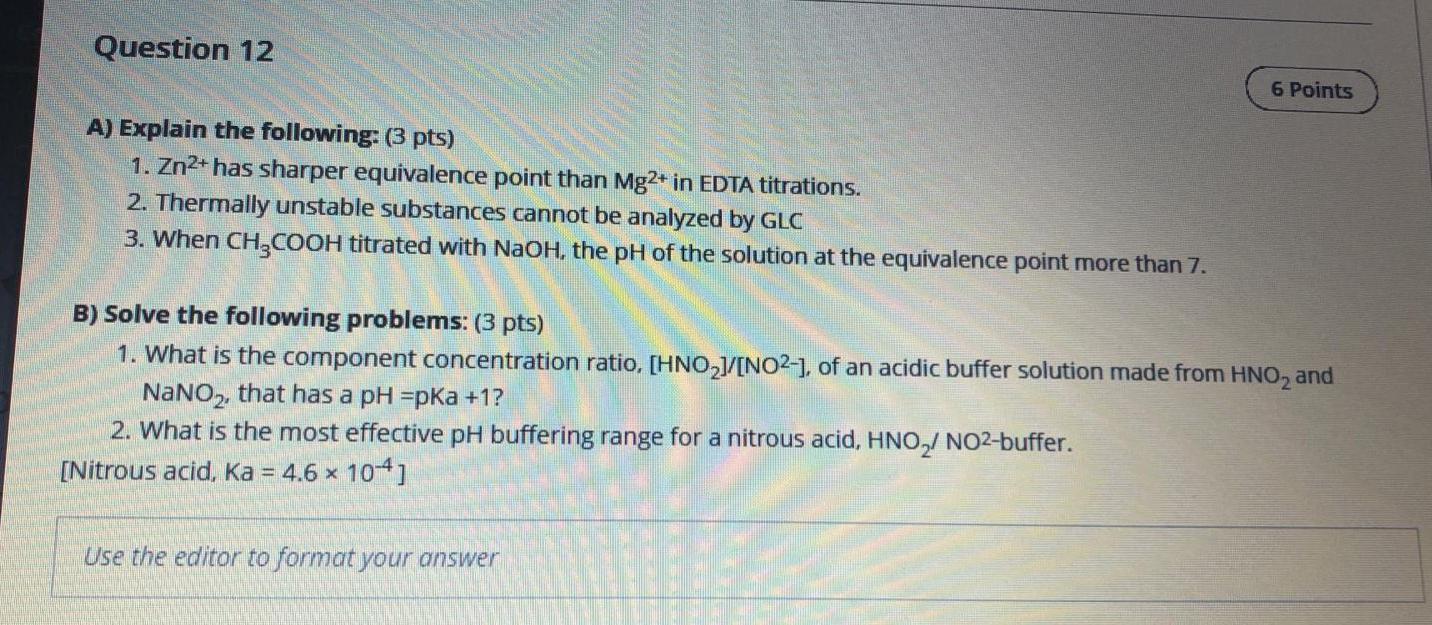
Question: I want it now please Question 12 6 Points A) Explain the following: (3 pts) 1. Zn2+ has sharper equivalence point than Mg2+ in EDTA
I want it now please
Question 12 6 Points A) Explain the following: (3 pts) 1. Zn2+ has sharper equivalence point than Mg2+ in EDTA titrations. 2. Thermally unstable substances cannot be analyzed by GLC 3. When CH3COOH titrated with NaOH, the pH of the solution at the equivalence point more than 7. B) Solve the following problems: (3 pts) 1. What is the component concentration ratio, [HNO2J/[NO2-), of an acidic buffer solution made from HNO2 and NaNO2, that has a pH =pKa +1? 2. What is the most effective pH buffering range for a nitrous acid, HNO, NO2-buffer. [Nitrous acid, Ka = 4.6 x 104] X Use the editor to format your
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


