Question: i want to solution step by step. This question about that Materials science engineering, metallurgical thermodynamics. 3. (30) Ammonia gas is heated to 300C. a)
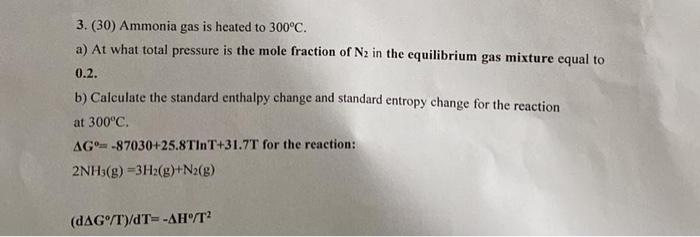
3. (30) Ammonia gas is heated to 300C. a) At what total pressure is the mole fraction of N2 in the equilibrium gas mixture equal to 0.2. b) Calculate the standard enthalpy change and standard entropy change for the reaction at 300C G=87030+25.8TT+31.7T for the reaction: 2NH3(g)=3H2(g)+N2(g) (dG/T)/dT=H/T2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


