Question: I would like someone to go through the steps on how exactly to solve this problem. I know the answer but need to know how
I would like someone to go through the steps on how exactly to solve this problem. I know the answer but need to know how to get there! Thanks!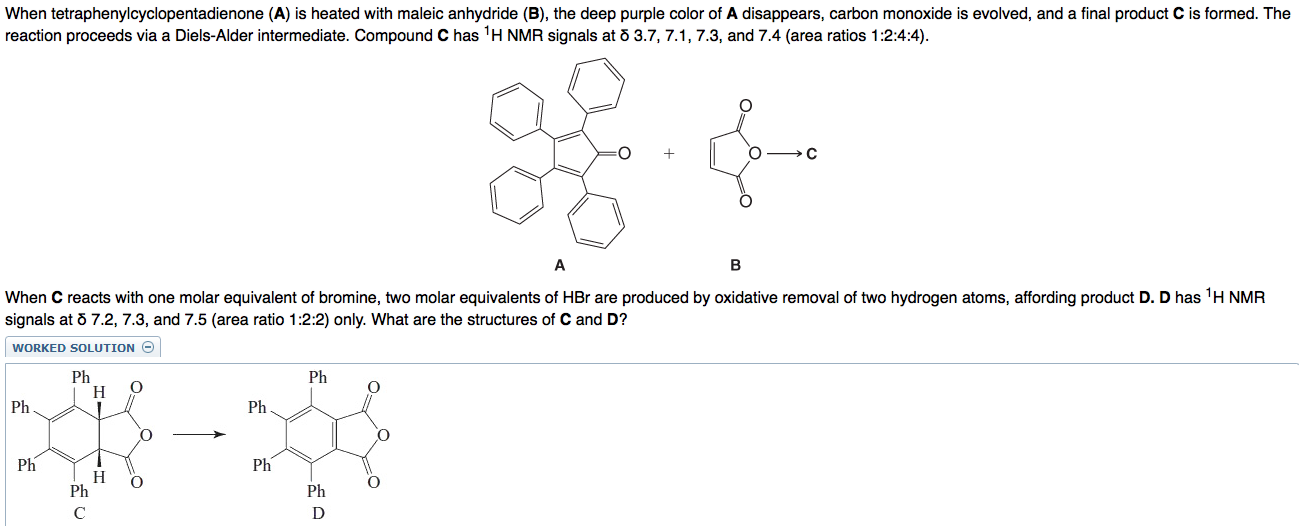
When tetraphenylcyclopentadienone (A) is heated with maleic anhydride (B), the deep purple color of A disappears, carbon monoxide is evolved, and a final product C is formed. The reaction proceeds via a Diels-Alder intermediate. Compound C has 'H NMR signals at 8 3.7, 7.1, 7.3, and 7.4 (area ratios 1:2:4:4). A B When C reacts with one molar equivalent of bromine, two molar equivalents of HBr are produced by oxidative removal of two hydrogen atoms, affording product D. D has 'H NMR signals at 8 7.2, 7.3, and 7.5 (area ratio 1:2:2) only. What are the structures of C and D? WORKED SOLUTION Ph 0 o Ph H 1 Ph Ph O 0 Ph Ph O O H Ph C Ph D
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


