Question: iClicker #5 (not for credit) A solution is created by mixing 500.mL of 1.50M CsOH(aq),250.mL of 1.00MHCl(aq) , and 250.mL of 1.00 MCH_(3)(C)/(O)O H(aq).K_(a)(CH_(3)(C)/(O)O H)=1.8times
iClicker #5 (not for credit)\ A solution is created by mixing
500.mLof
1.50M
CsOH(aq),250.mLof
1.00MHCl(aq), and
250.mLof 1.00
MCH_(3)(C)/(O)O H(aq).K_(a)(CH_(3)(C)/(O)O H)=1.8\\\\times 10^(-5)\ After mixing, which species has the dominant effect on the
pHof the solution that remains?\ A.
HCl(aq)\ B.
CH_(3)(C)/(O)O H(aq)\ C.
CsOH(aq)\ D.
CsCl(aq)\ E.
CH_(3)(C)/(O)O Cs(aq) 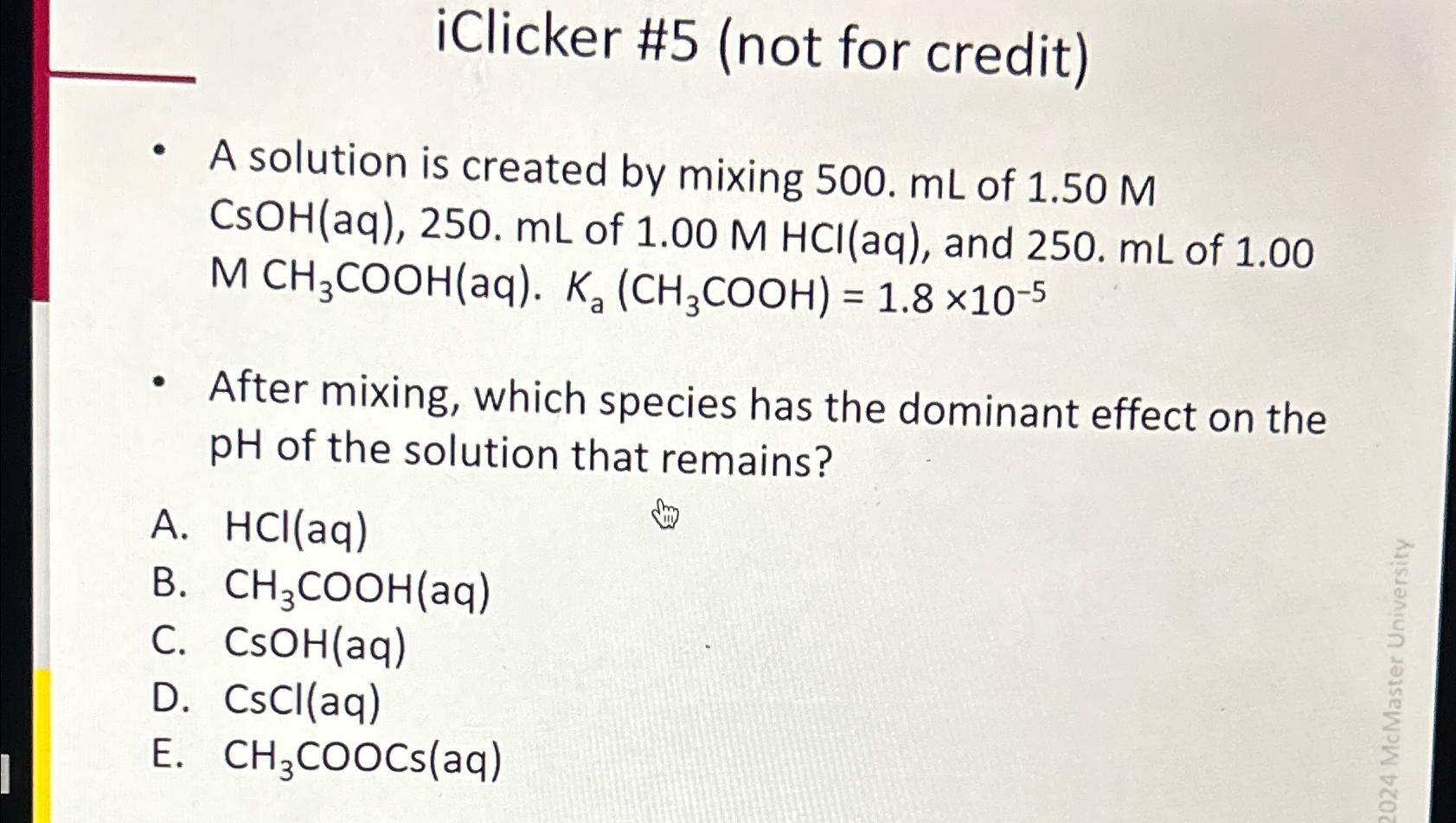
- A solution is created by mixing 500.mL of 1.50M CsOH(aq),250.mL of 1.00MHCl(aq), and 250.mL of 1.00 MCH3COOH(aq).Ka(CH3COOH)=1.8105 - After mixing, which species has the dominant effect on the pH of the solution that remains? A. HCl(aq) B. CH3COOH(aq) C. CsOH(aq) D. CsCl(aq) E. CH3COOCs(aq) - A solution is created by mixing 500.mL of 1.50M CsOH(aq),250.mL of 1.00MHCl(aq), and 250.mL of 1.00 MCH3COOH(aq).Ka(CH3COOH)=1.8105 - After mixing, which species has the dominant effect on the pH of the solution that remains? A. HCl(aq) B. CH3COOH(aq) C. CsOH(aq) D. CsCl(aq) E. CH3COOCs(aq)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


