Question: Idensty limiting meactants (mavimum product method). Close Problem Consider the reaction of manganese dioxide with potassium hydroxide and oxygen. 2MnO2(s)+4KOH(aq)+O2(g)2K2MnO4(aq)+2H2O(l) Determine the limiting reactant in
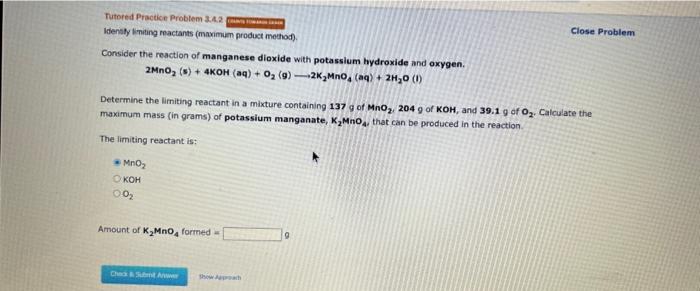
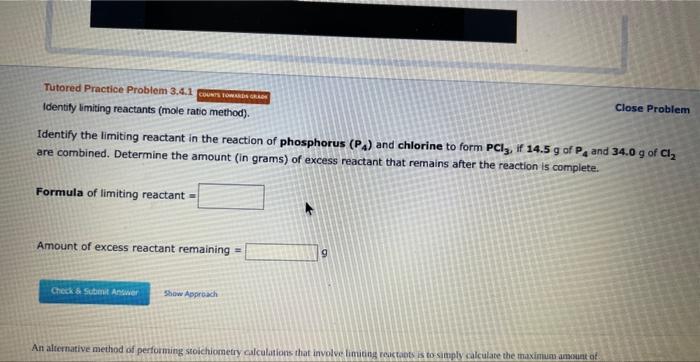
Idensty limiting meactants (mavimum product method). Close Problem Consider the reaction of manganese dioxide with potassium hydroxide and oxygen. 2MnO2(s)+4KOH(aq)+O2(g)2K2MnO4(aq)+2H2O(l) Determine the limiting reactant in a mixture containing 137g of MnO2,204g of KOH,and39.1g of O2. Calculate the maximum mass (in grams) of potassium manganate, K2MnO4, that can be produced in the reaction. The limiting reactant is: MnO2KOHO2 Amount of K2MnO4 forrned = 9 Identify the limiting reactant in the reaction of phosphorus (P4) and chlorine to form PCl3, if 14.5g of P4 and 34.0g of Cl2 are combined. Determine the amount (in grams) of excess reactant that remains after the reaction is complete
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


