Question: Identify the molecule represented by the Mass Spectrum below. MASS SPECTRUM 100- 80 60 40 20 0.0 30 60 45 m/z Rel. Intensity 0.0
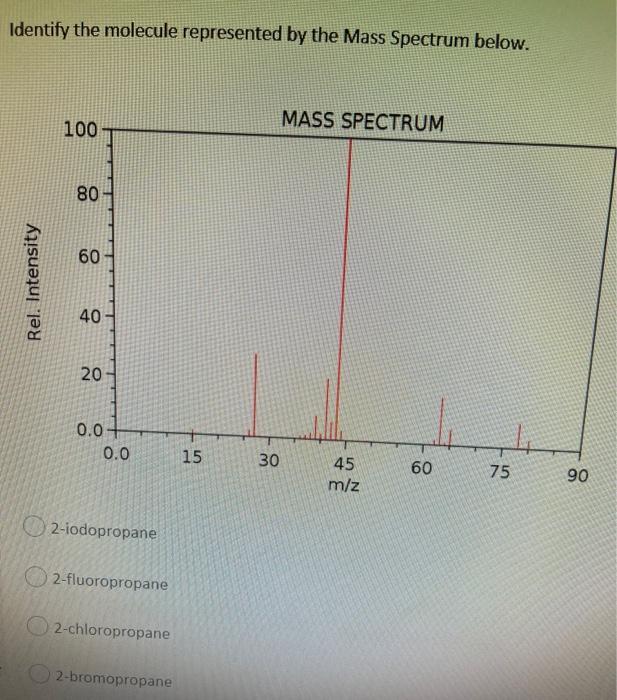
Identify the molecule represented by the Mass Spectrum below. MASS SPECTRUM 100- 80 60 40 20 0.0 30 60 45 m/z Rel. Intensity 0.0 2-iodopropane 2-fluoropropane 2-chloropropane 2-bromopropane 15 75 90
Step by Step Solution
3.44 Rating (154 Votes )
There are 3 Steps involved in it
Here the molecular weight of the compound is near 60 Thats why its bas... View full answer

Get step-by-step solutions from verified subject matter experts


