Question: Identify the van't Hoff factors for FP sample 1, which is an organic compound, and FP sample 2, which is an ionic compound. van't Hoff

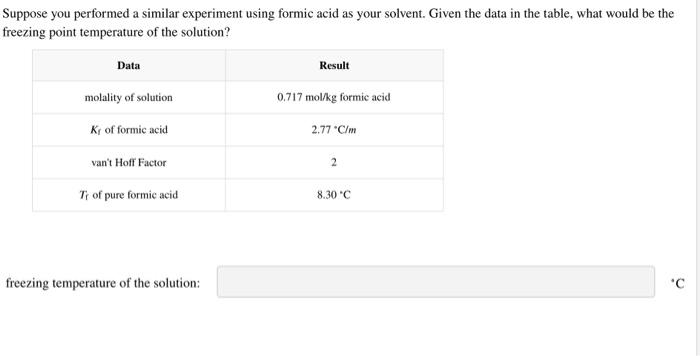
Identify the van't Hoff factors for FP sample 1, which is an organic compound, and FP sample 2, which is an ionic compound. van't Hoff factor for FP sample 1: van't Hoff factor for FP sample 2: Suppose you performed a similar experiment using formic acid as your solvent. Given the data in the table, what would be the freezing point temperature of the solution
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


