Question: If a solution containing Ag and HNO3 are mixed. Will the resulting compound be soluble? Yes O No QUESTION 15 Ifa solution containing Ba2+ and
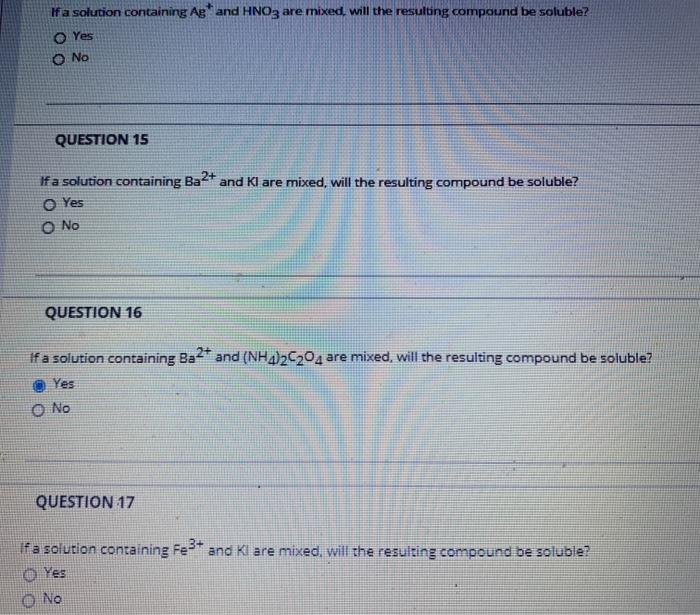

If a solution containing Ag and HNO3 are mixed. Will the resulting compound be soluble? Yes O No QUESTION 15 Ifa solution containing Ba2+ and I are mixed, will the resulting compound be soluble? Yes O No QUESTION 16 If a solution containing Ba2+ and (NH4)2C2O4 are mixed, will the resulting compound be soluble? Yes O No QUESTION 17 if a solution containing Fe 3+ and Kl are mixed will the resulting compound be soluble? Yes No If a solution containing Ag* and Na2CO3 are mixed, will the resulting compound be soluble? O Yes No QUESTION 19 If a solution containing Ba2+ and KSCN are mixed, will the resulting compound be soluble? O Yes O No QUESTION 20 If a solution containing Fe3+ and H2504 are mixed, will the resulting compound be soluble? O Yes
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


