Question: If an expert can please answer page 50 number 1, 2, and 3 by the end of the day today Tuesday January 31, 2023 or


If an expert can please answer page 50 number 1, 2, and 3 by the end of the day today Tuesday January 31, 2023 or as soon as possible please and thank you in advance. This is for Chem 65.
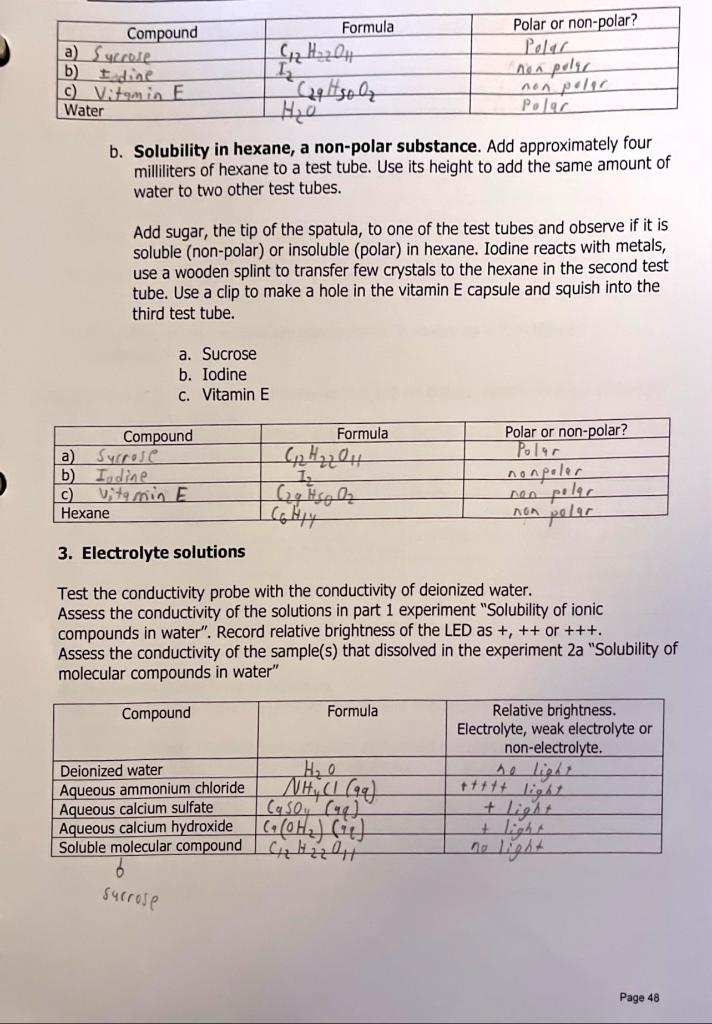
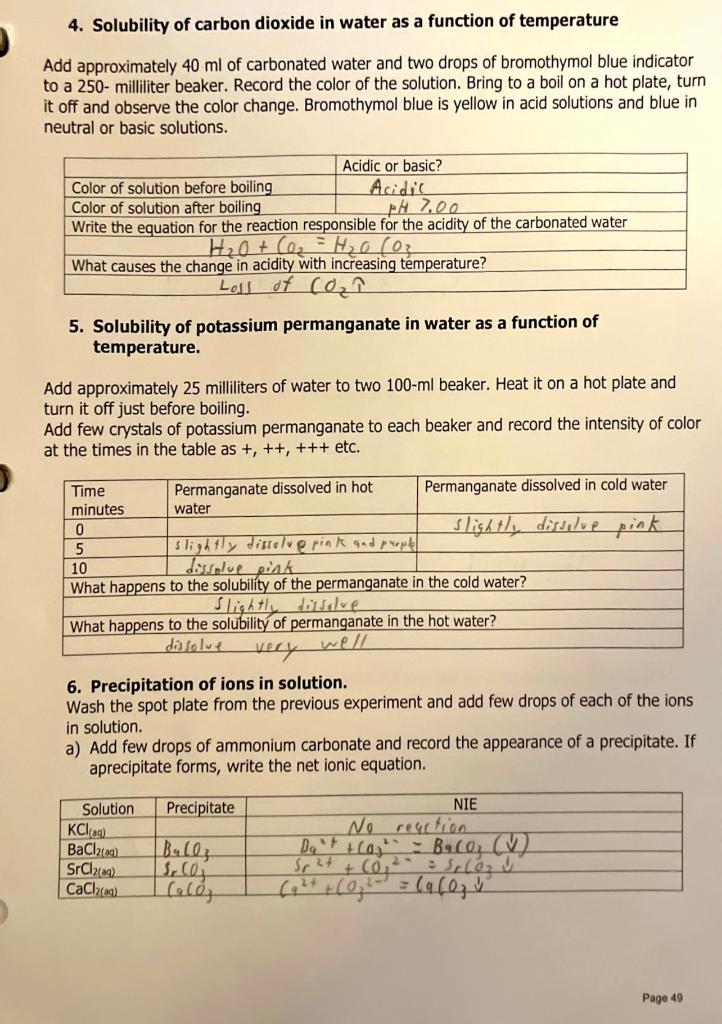
Purpose: Study the property of ionic and molecular compounds Procedure 1. Solubility of ionic compounds in water Add approximately 4 milliliters of water to a test tube. Use its height to add the same amount of water to two other test tubes. Add the tip of the spatula of each of the solids to different test tubes. Add more or less the same amount to each one. Mix the contents of the test tubes tapping the bottom of the test tub with the thumb. Observe if it is soluble, insoluble or slightly soluble. Save the samples for the conductivity test. b. Ammonium chloride c. Calcium sulfate d. Calcium hydroxide 2. Polarity of molecular compounds a. Solubility in water, a polar substance. Add approximately 4 milliliters of water to a test tube. Use its height to add the same amount of water to two other test tubes. Add sugar, the tip of the spatula, to one of the test tubes. Mix and observe if it is soluble (polar) or insoluble (non-polar). Iodine reacts with metals, use a wooden splint to transfer few crystals to the water in the second test tube. Use a clip to make a hole in the vitamin E capsule and squish into the third test tube. Save the test tube(s) with solutions (dissolved substances) for the conductivity test. a. Sucrose b. Iodine c. Vitamin E b. Solubility in hexane, a non-polar substance. Add approximately four milliliters of hexane to a test tube. Use its height to add the same amount of water to two other test tubes. Add sugar, the tip of the spatula, to one of the test tubes and observe if it is soluble (non-polar) or insoluble (polar) in hexane. Iodine reacts with metals, use a wooden splint to transfer few crystals to the hexane in the second test tube. Use a clip to make a hole in the vitamin E capsule and squish into the third test tube. a. Sucrose b. Iodine c. Vitamin E 3. Electrolyte solutions Test the conductivity probe with the conductivity of deionized water. Assess the conductivity of the solutions in part 1 experiment "Solubility of ionic compounds in water". Record relative brightness of the LED as +,++ or +++. Assess the conductivity of the sample(s) that dissolved in the experiment 2a "Solubility of molecular compounds in water" 4. Solubility of carbon dioxide in water as a function of temperature Add approximately 40ml of carbonated water and two drops of bromothymol blue indicator to a 250 - milliliter beaker. Record the color of the solution. Bring to a boil on a hot plate, turn it off and observe the color change. Bromothymol blue is yellow in acid solutions and blue in neutral or basic solutions. 5. Solubility of potassium permanganate in water as a function of temperature. Add approximately 25 milliliters of water to two 100ml beaker. Heat it on a hot plate and turn it off just before boiling. Add few crystals of potassium permanganate to each beaker and record the intensity of color at the times in the table as +,++,+++ etc. 6. Precipitation of ions in solution. Wash the spot plate from the previous experiment and add few drops of each of the ions in solution. a) Add few drops of ammonium carbonate and record the appearance of a precipitate. If aprecipitate forms, write the net ionic equation. 1. Are your results in parts 2a and 2b consistent? i.e. Do they provide the same results? Explain. 2. Are your results in parts 1 and 3 consistent? Explain. 3. In the flame test, different colors of light are emitted by different ions. What is the source of the light emitted
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


