Question: If ideal temperature rise during compression for a reversible and adiabatic process is . By specifying efficiency , irreversibility can be handled. How can it
If ideal temperature rise during compression for a reversible and adiabatic process is 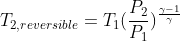 . By specifying efficiency , irreversibility can be handled. How can it be shown that the actual temperature rise during compression can be calculated using:
. By specifying efficiency , irreversibility can be handled. How can it be shown that the actual temperature rise during compression can be calculated using: 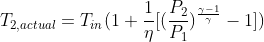 ?
?
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


