Question: if possible could u please explain also :) i have part i done so if you could start at ii i would appriciate it Discuss
if possible could u please explain also :)
i have part i done so if you could start at ii i would appriciate it
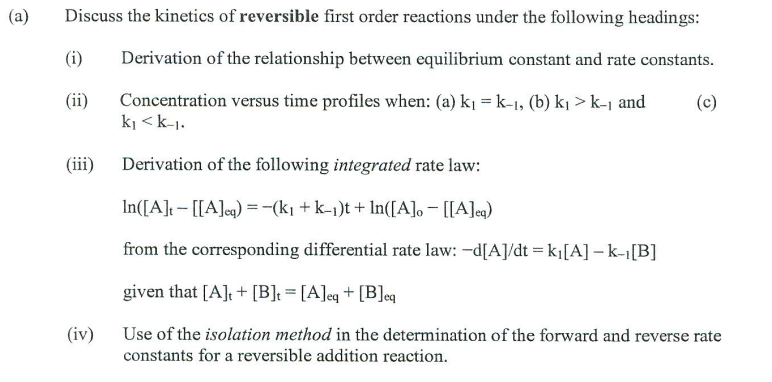
Discuss the kinetics of reversible first order reactions under the following headings: (i) Derivation of the relationship between equilibrium constant and rate constants. (ii) Concentration versus time profiles when: (a) k1=k1, (b) k1>k1 and (c) k1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


