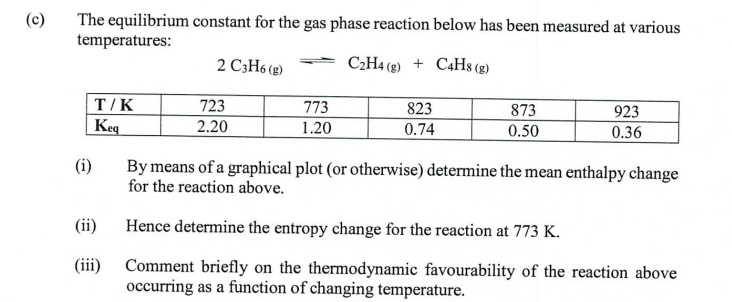
Question: if possible could u please explain also :) The equilibrium constant for the gas phase reaction below has been measured at various temperatures: 2C3H6(g)C2H4(g)+C4H8(g) (i)
 if possible could u please explain also :)
if possible could u please explain also :)
The equilibrium constant for the gas phase reaction below has been measured at various temperatures: 2C3H6(g)C2H4(g)+C4H8(g) (i) By means of a graphical plot (or otherwise) determine the mean enthalpy change for the reaction above. (ii) Hence determine the entropy change for the reaction at 773K. (iii) Comment briefly on the thermodynamic favourability of the reaction above occurring as a function of changing temperature
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


