Question: If this reaction is initially at equilibrium, predict what will happen when the system is altered in the followimg ways. Given the reaction: CO2(g)+2NH3(g)CO(NH2)2(g)+H2O(g)Kp=0.721at500K If
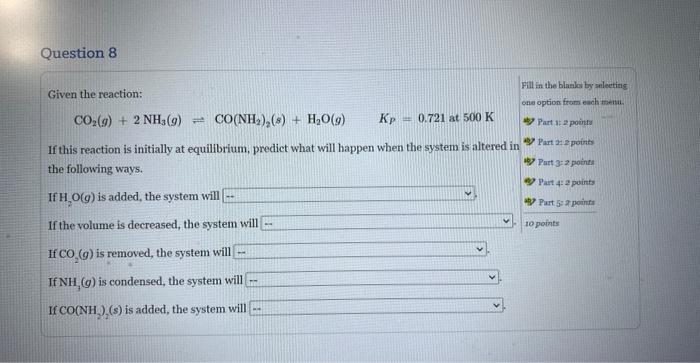
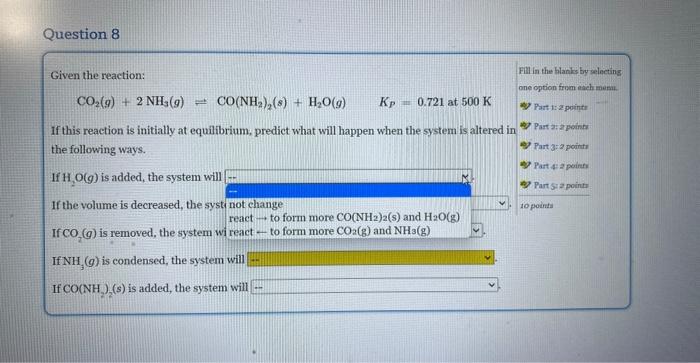
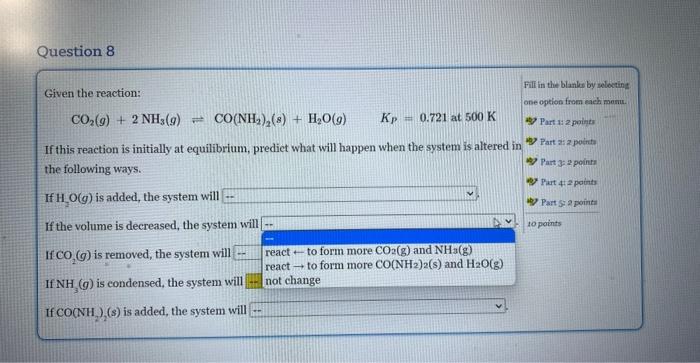
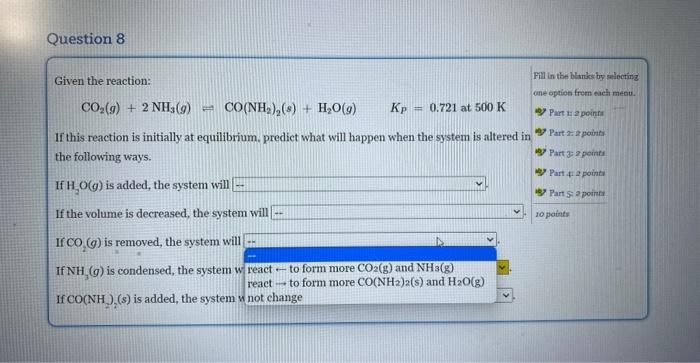
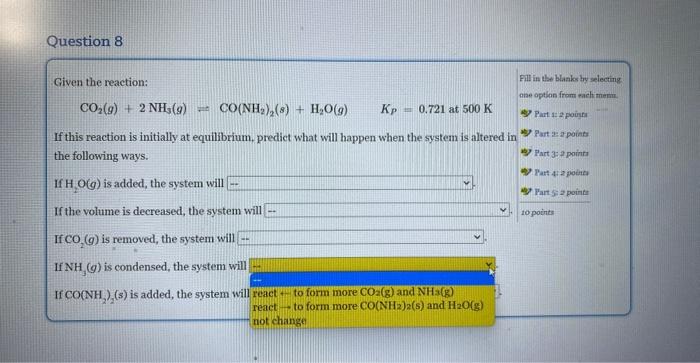
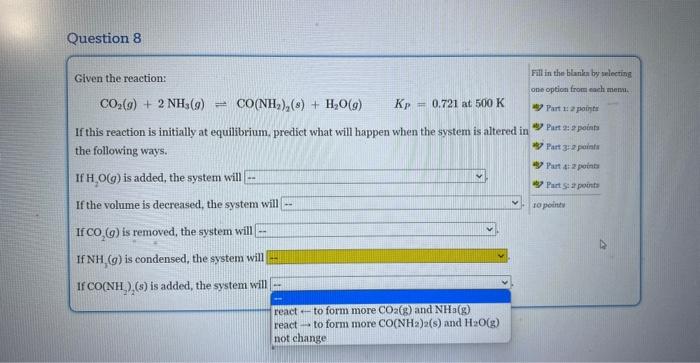
Given the reaction: CO2(g)+2NH3(g)CO(NH2)2(g)+H2O(g)Kp=0.721at500K If this reaction is initially at equilibrium, predict what will happen when the system is altered in the following ways. If H2O(g) is added, the system will If the volume is decreased, the system will If CO2(g) is removed, the system will If NH3(g) is condensed, the system will If CO(NH2)2(s) is added, the system will Given the reaction: CO2(g)+2NH3(g)=CO(NH2)2(s)+H2O(g)Kp=0.721at500K If this reaction is initially at equilibrium, predict what will happen when the system is altered in the following ways. If H2O(g) is added, the system will If the volume is decreased, the syst not change reacttoformmoreCO(NH2)2(s)andH2O(g) If CO2(g) is removed, the system wi react - to form more CO2(g) and NH3(g) If NH3(g) is condensed, the system will If CO(NH2)2(s) is added, the system will Given the reaction: CO2(g)+2NH3(g)CO(NH2)2(s)+H2O(g)KP=0.721at500K If this reaction is initially at equilibrium, prediet what will happen when the system is altered in the following ways. If H2O(g) is added, the system will If the volume is decreased, the system will If CO2(g) is removed, the system will If NH3(g) is condensed, the system will \left. If \( {\mathrm{CO}\left(\mathrm{NH}_{2} ight.}_{2} ight)_{2}(s) \) is added, the system will Given the reaction: CO2(g)+2NH3(g)=CO(NH2)2(s)+H2O(g)KP=0.721at500K If this reaction is initially at equilibrium, predict what will happen when the system is altered in the following ways. If H2O(g) is added, the system will If the volume is decreased, the system will If CO2(g) is removed, the system will If NH3(g) is condensed, the system w If CO(NH2)2(s) is added, the system v Given the reaction: CO2(g)+2NH3(g)=CO(NH2)2(s)+H2O(g)Kp=0.721at500K If this reaction is initially at equilibrium, predict what will happen when the system is altered in the following ways. If H2O(g) is added, the system will If the volume is decreased, the system will If CO2(g) is removed, the system will. If NH3(g) is condensed, the system will If CO(NH2)2(s) is added, the system wil Given the reaction: CO2(g)+2NH3(g)=CO(NH2)2(s)+H2O(g)Kp=0.721at500K If this reaction is initially at equilibrium, predict what will happen when the system is altered in the following ways. If H2O(g) is added, the system will If the volume is decreased, the system will If CO2(g) is removed, the system will If NH3(g) is condensed, the system will If CO(NH2)2(s) is added, the system will
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


