Question: If this reaction system is initially at equilibrium and a catalyst is added, the new position of equilibrium will be Given the reaction: Fill in
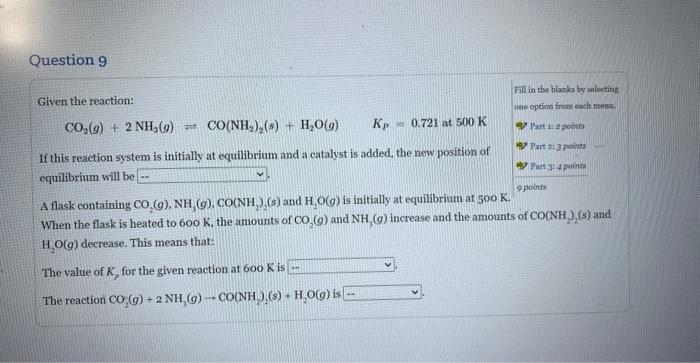
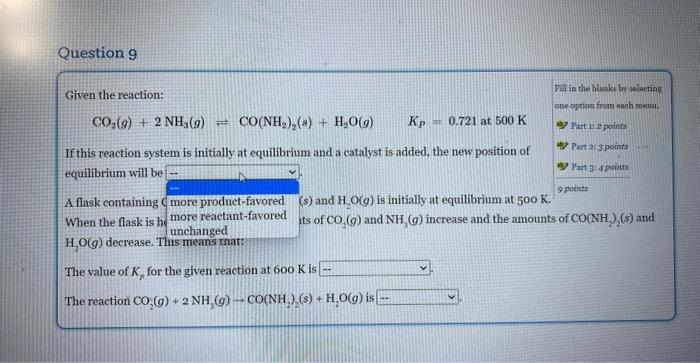
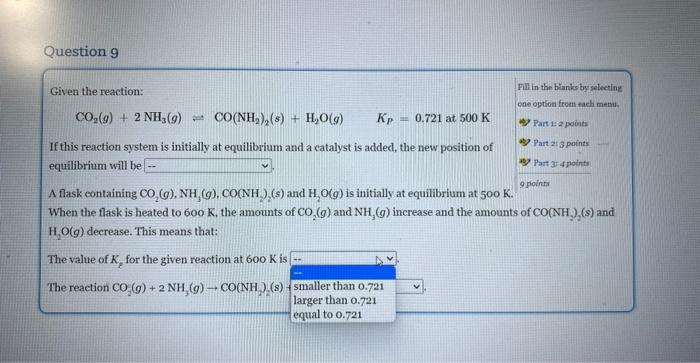
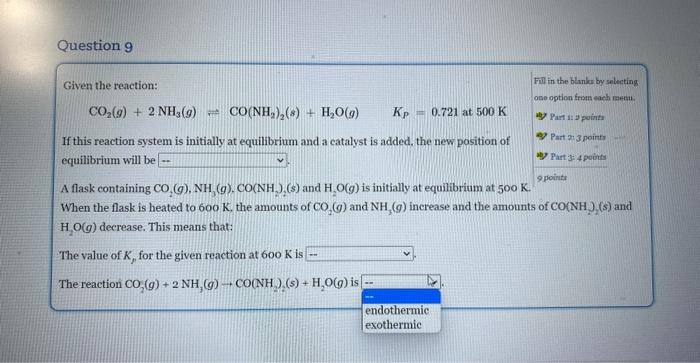
Given the reaction: Fill in the blanks by selecting CO2(g)+2NH3(g)CO(NH2)2(s)+H2O(g)Kp=0.721at500K ane eption from each nsmts. If this reaction system is initially at equilibrium and a catalyst is added, the new position of equilibrium will be A flask containing CO2(g),NH3(g),CO(NH2)2(s) and H2O(g) is initially at equilibrium at 500K. Part is 2 points Rare 2:3 pounts When the flask is heated to 600K, the amounts of CO2(g) and NH3(g) increase and the amounts of CO2(NH2)2(s) and H2O(g) decrease. This means that: The value of Kp for the given reaction at 600K is The reaction CO2(g)+2NH3(g)CO(NH3)(s)+H2O(g) is Given the reaction: Fili is the blanks by seloeting CO2(g)+2NH3(g)CO(NH2)2(s)+H2O(g)KP=0.721at500K obe option from elah mena, If this reaction system is initially at equilibrium and a catalyst is added, the new position of equilibrium will be A flask containing (s) and H2O(g) is initially at equilibrium at 500K. When the flask is h ts of CO2(g) and NH3(g) increase and the amounts of CO(NH2)(s) and H2O(g) decrease. T The value of Kp for the given reaction at 600K is The reaction CO2(g)+2NH3(g)CO(NH2)2(s)+H2O(g) is Given the reaction: Fill in the blanks by selecting CO2(g)+2NH3(g)=CO2(NH2)2(s)+H2O(g)Kp=0.721at500K cne option from rach menm. If this reaction system is initially at equilibrium and a eatalyst is added, the new position of Part 1 a points equilibrium will be A flask containing CO2(g),NH3(g),CO2NH2)2(s) and H2O(g) is initially at equilibrium at 50K. When the flask is heated to 600K, the amounts of CO2(g) and NH3(g) increase and the amounts of CO2(NH2)2(s) and H2O(g) decrease. This means that: The value of Kp for the given reaction at 600K is The reaction CO2(g)+2NH3(g)CO2NH2(s) Given the reaction: Fill in the Blanke by selecting CO2(g)+2NH3(g)CO2(NH2)2(g)+H2O(g)Kp=0.721at500K ono option froma warh matad. ab) Part i: i pointa If this reaction system is initially at equilibrium and a catalyst is added, the new position of 4) Part 2 ipoints equilibrium will be A flask containing CO2(g),NH3(g),CO(NH2)2(s) and H2O(g) is initially at equilibrium at 500K. ver Part 3 : 4 points 2pointe When the flask is heated to 600K, the amounts of CO2(g) and NH3(g) increase and the amounts of CO(NH2)2(s) and H2O(g) decrease. This means that: The value of Kp for the given reaction at 600K is The reaction CO2(g)+2NH3(g)+CO(NH22(s)+H2O(g) is
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


