Question: If you use Euler method to calculate the concentrations at each time step, please explain how you calculate. 2. (50 points) Partial oxidation of cholesterol
 If you use Euler method to calculate the concentrations at each time step, please explain how you calculate.
If you use Euler method to calculate the concentrations at each time step, please explain how you calculate.
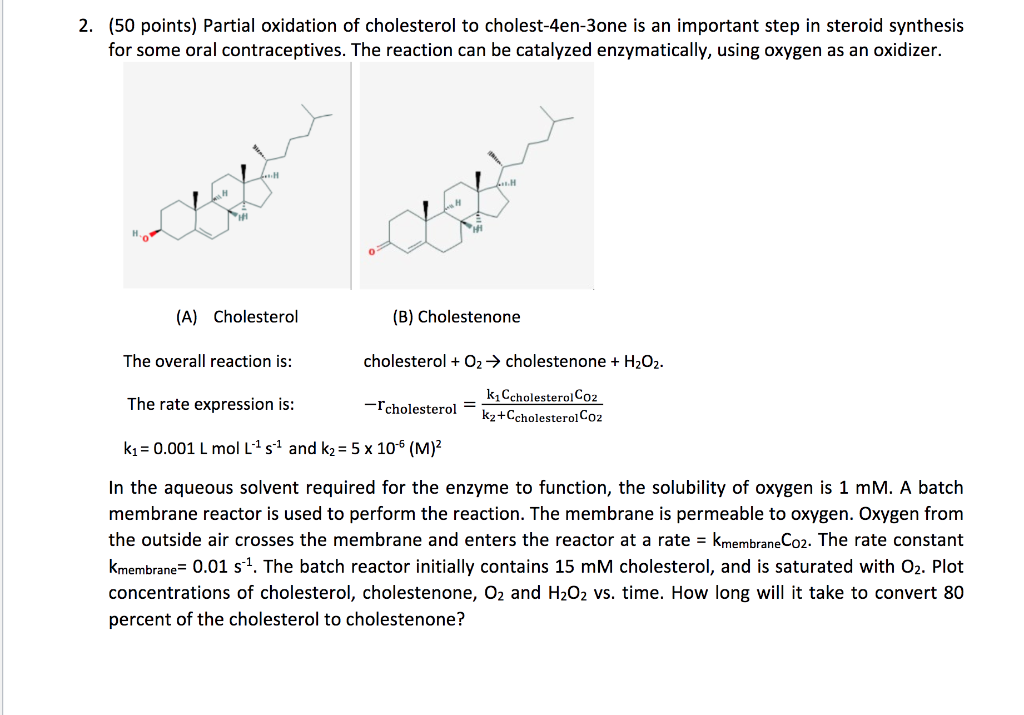
2. (50 points) Partial oxidation of cholesterol to cholest-4en-3one is an important step in steroid synthesis for some oral contraceptives. The reaction can be catalyzed enzymatically, using oxygen as an oxidizer. (A) Cholesterol (B) Cholestenone The overall reaction is: cholesterol +O2 cholestenone +H2O2. The rate expression is: rcholesterol=k2+CcholesterolC02k1CcholesterolC02 k1=0.001LmolL1s1 and k2=5106(M)2 In the aqueous solvent required for the enzyme to function, the solubility of oxygen is 1mM. A batch membrane reactor is used to perform the reaction. The membrane is permeable to oxygen. Oxygen from the outside air crosses the membrane and enters the reactor at a rate =kmembraneC02. The rate constant kmembrane=0.01s1. The batch reactor initially contains 15mM cholesterol, and is saturated with O2. Plot concentrations of cholesterol, cholestenone, O2 and H2O2 vs. time. How long will it take to convert 80 percent of the cholesterol to cholestenone
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


