Question: II- ,NO :NO ( ) N2O4. cm 7.1 cm 10 Opening the cock between the two vessels in the figure, the two gases mix to
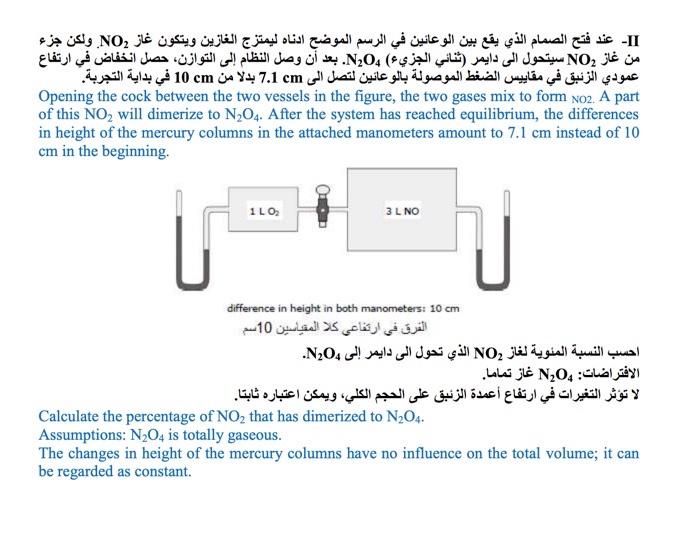
II- ,NO :NO ( ) N2O4. cm 7.1 cm 10 Opening the cock between the two vessels in the figure, the two gases mix to form No2. A part of this NO2 will dimerize to N204. After the system has reached equilibrium, the differences in height of the mercury columns in the attached manometers amount to 7.1 cm instead of 10 cm in the beginning. 110 3L NO difference in height in both manometers: 10 cm 10 ,NO N20. : N0 . Calculate the percentage of NO2 that has dimerized to N204. Assumptions: N204 is totally gaseous. The changes in height of the mercury columns have no influence on the total volume; it can be regarded as constant
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


