Question: ii. Using the inversion operator i, determine the g or u symmetries for the (5, TI: and 5 orbitals. (Hint: it is easiest to do
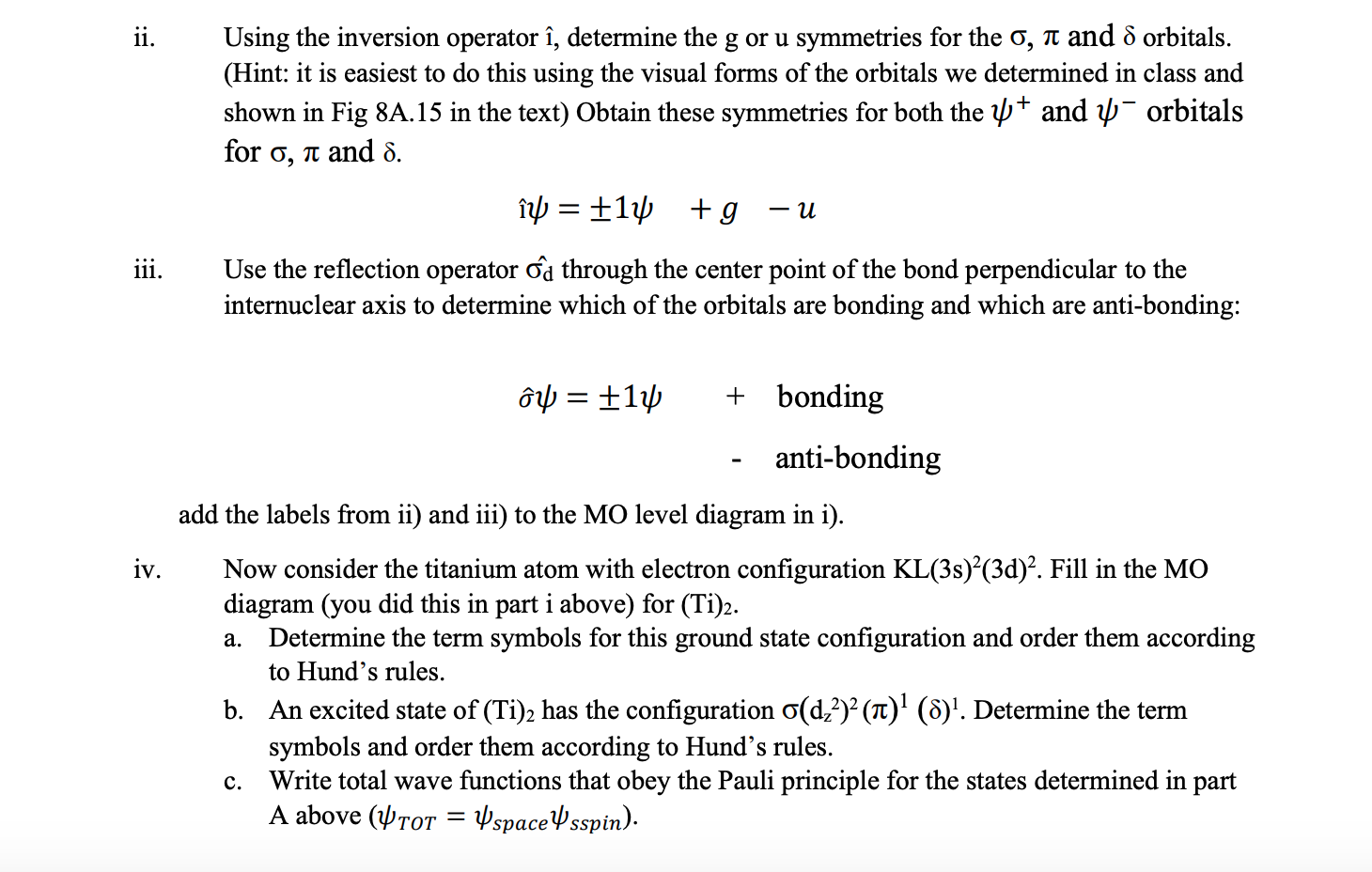
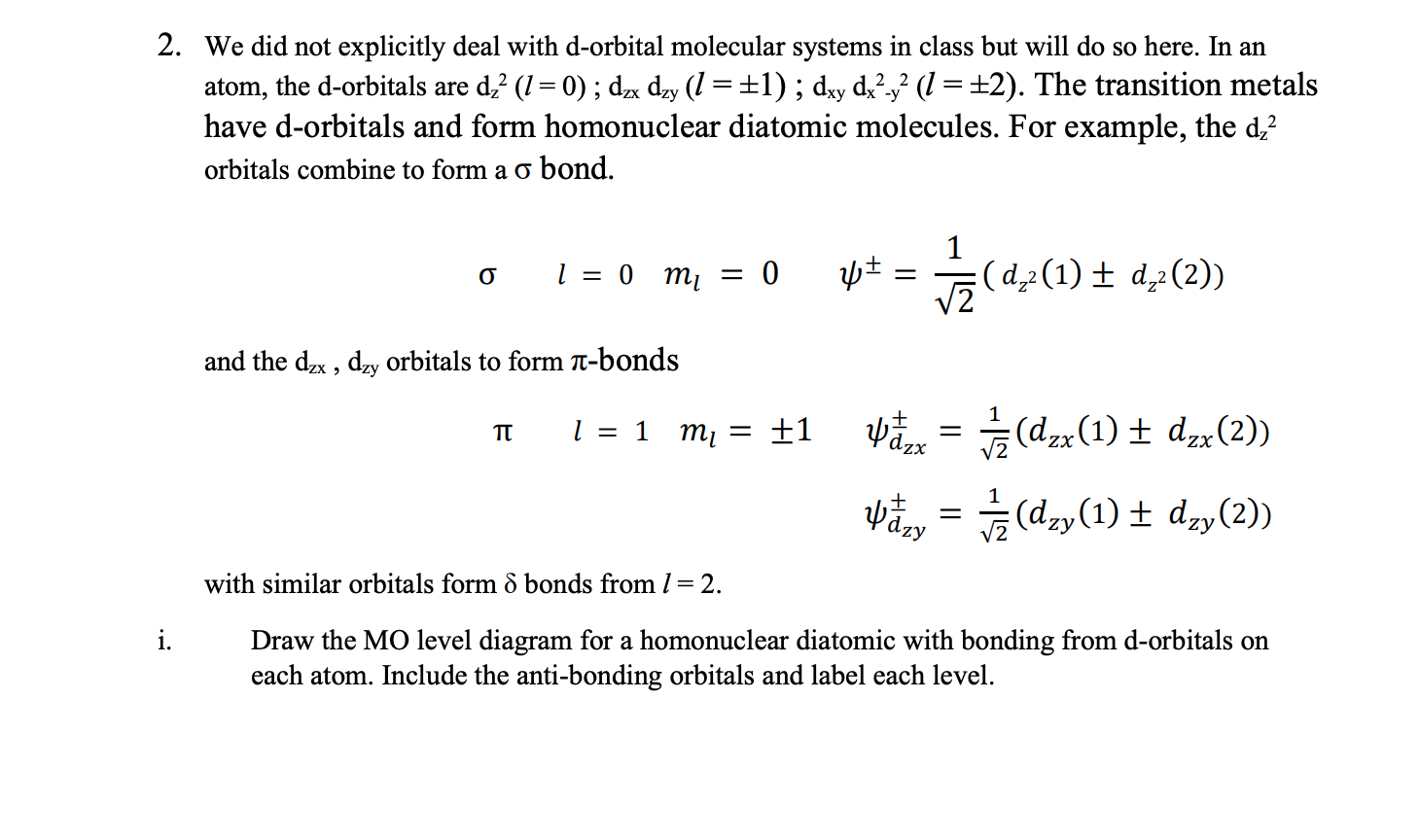
ii. Using the inversion operator i, determine the g or u symmetries for the (5, TI: and 5 orbitals. (Hint: it is easiest to do this using the visual forms of the orbitals we determined in class and shown in Fig 8A.15 in the text) Obtain these symmetries for both the IIJ+ and 11f orbitals for o, a and 5. izp=i11p +g u iii. Use the reection operator dd through the center point of the bond perpendicular to the internuclear axis to determine which of the orbitals are bonding and which are anti-bonding: 61/) = i 11]) + bonding - anti-bonding add the labels from ii) and iii) to the MO level diagram in i). iv. Now consider the titanium atom with electron conguration KL(3 s)2(3d)2. Fill in the MO diagram (you did this in part i above) for (Ti)2. a. Determine the term symbols for this ground state conguration and order them according to Hund's rules. b. An excited state of (Ti); has the conguration (5(d22)2 (71:)1 (8)1. Determine the term symbols and order them according to Hund's rules. c. Write total wave functions that obey the Pauli principle for the states determined in part A above (wTOT = wspacelpsspin) 2. We did not explicitly deal with d-orbital molecular systems in class but will do so here. In an atom, the d-orbitals are dz? (1 = 0) ; dzx dzy (1 = +1) ; dxy dx2y? (1 = +2). The transition metals have d-orbitals and form homonuclear diatomic molecules. For example, the dz orbitals combine to form a o bond. 1 =0 m = 0 VZ (d,2 (1) + d,2 (2)) and the dzx , dzy orbitals to form -bonds TT 1 = 1 m = +1 Udzx = (dzx (1) + dzx (2)) Udzy = (dzy (1) + dzy(2)) with similar orbitals form 8 bonds from / = 2. i. Draw the MO level diagram for a homonuclear diatomic with bonding from d-orbitals on each atom. Include the anti-bonding orbitals and label each level
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


