Question: iii ) ( From an old Thermo 1 exam ) : A horizontal cylinder contains an explosive gas and an inert gas separated by a
iiiFrom an old Thermo exam: A horizontal cylinder contains an explosive gas and an inert
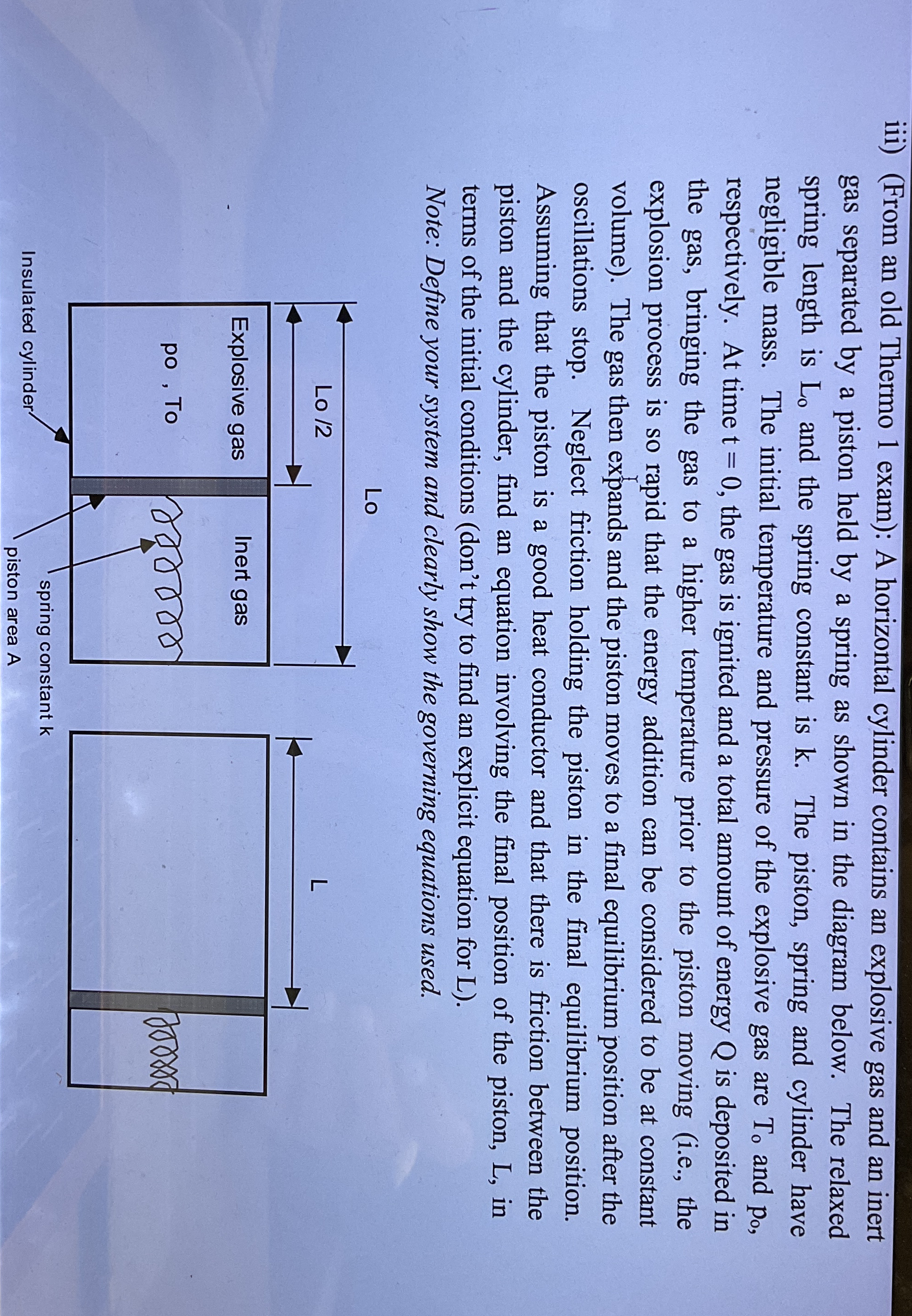
gas separated by a piston held by a spring as shown in the diagram below. The relaxed
spring length is and the spring constant is The piston, spring and cylinder have
negligible mass. The initial temperature and pressure of the explosive gas are and
respectively. At time the gas is ignited and a total amount of energy is deposited in
the gas, bringing the gas to a higher temperature prior to the piston moving ie the
explosion process is so rapid that the energy addition can be considered to be at constant
volume The gas then expands and the piston moves to a final equilibrium position after the
oscillations stop. Neglect friction holding the piston in the final equilibrium position.
Assuming that the piston is a good heat conductor and that there is friction between the
piston and the cylinder, find an equation involving the final position of the piston, L in
terms of the initial conditions dont try to find an explicit equation for L
Note: Define your system and clearly show the governing equations used.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


