Question: III. LARGER MOLECULES Most molecules, such as the one below, are much larger than the ones we have con-: sidered so far. They do not
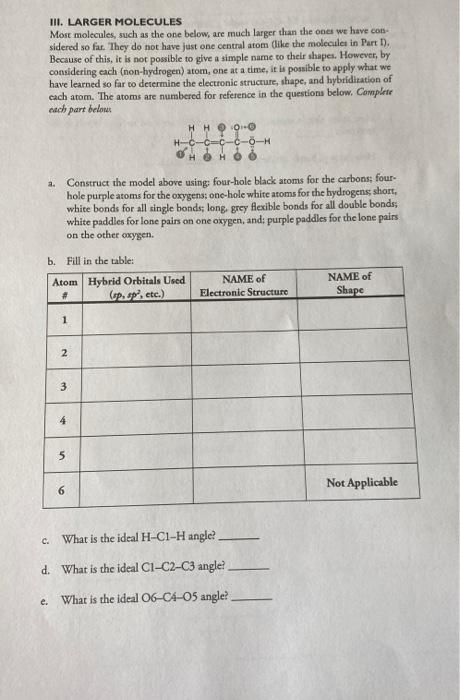
III. LARGER MOLECULES Most molecules, such as the one below, are much larger than the ones we have con-: sidered so far. They do not have just one central atom (tike the molecules in Part D). Because of this, it is not possible to give a simple name to their shapes. However, by considering each (non-hydrogen) atom, one at a time, it is possible to apply what we have learned so far to derermine the electronic structure, shape, and hybriditation of each atom. The atoms are numbered for reference in the questions below. Conplare cach part belows a. Construct the model above using; four-hole black atoms for the carbons; fourhole purple atoms for the oxygens; onc-hole white atoms for the hydrogens short, whice bonds for all single bonds; long, grey flexible bonds for all double bonds; white paddles for lone pairs on one oxygen, and: purple paddles for the lone pairs. on the other axygen. h Fill in the tahle: c. What is the ideal HClH angle? d. What is the ideal ClC2C3 angle? e. What is the ideal O6COa angle
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


