Question: es Three of the reactions that occur when the paraffin of a candle (typical formula C21H44) burns are as follows: (1) Complete combustion forms

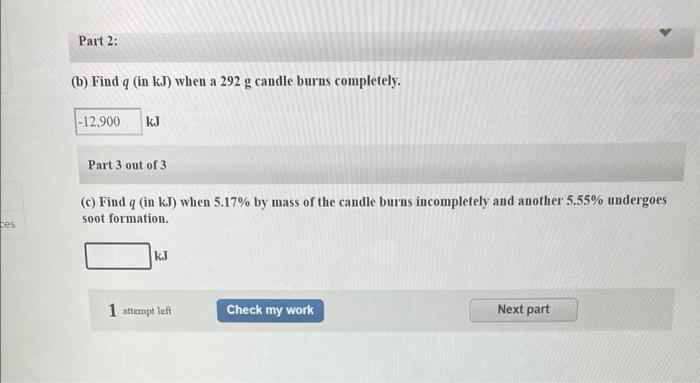
es Three of the reactions that occur when the paraffin of a candle (typical formula C21H44) burns are as follows: (1) Complete combustion forms CO and water vapor. (2) Incomplete combustion forms CO and water vapor. (3) Some wax is oxidized to elemental C (soot) and water vapor. Part 1: 0 (a) Find AH of each reaction for 1 mol of C21H44 (AH of CH44-476 kJ/mol; use combustion graphite for elemental carbon). (1) -13,108 kJ 3 (2) -7.165 kJ E (3) -4,844 kJ ces Part 2: (b) Find q (in kJ) when a 292 g candle burns completely. -12,900 kJ Part 3 out of 3 (c) Find q (in kJ) when 5.17% by mass of the candle burns incompletely and another 5.55% undergoes soot formation. kJ attempt left Check my work Next part
Step by Step Solution
3.42 Rating (142 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


