Question: imwhat is the right answer for q4 and 5 QUESTION 4 10 poin Calculate the amount of heat that must be removed by an industrial
imwhat is the right answer for q4 and 5 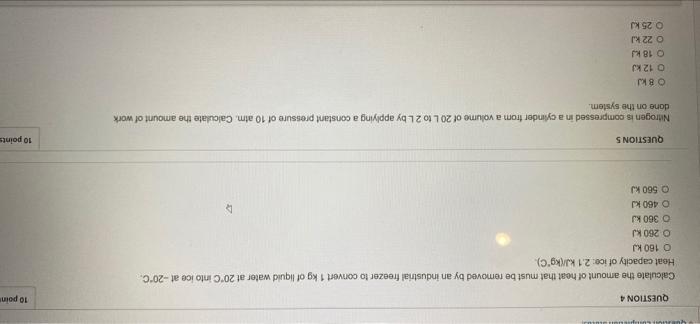

QUESTION 4 10 poin Calculate the amount of heat that must be removed by an industrial freezer to convert 1 kg of liquid water at 20C into ice at -20C. Heat capacity of ice: 2.1 kJ/kg C) O 160 kJ 0 280 kJ O 360 kJ O 460 kJ O 560 kJ 10 points QUESTIONS Nitrogen is compressed in a cylinder from a volume of 20 L to 2 Lby applying a constant pressure of 10 atm Calculate the amount of work done on the system O 8 kJ 0 12 kJ O 18 kJ O 22 kJ 25 kJ
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


