Question: In 9 Days 0 General Scien... Fuad Hassan - CA General Science 2 A Tier Practice: 115% Balance the following equation: Al4C3 (s) +
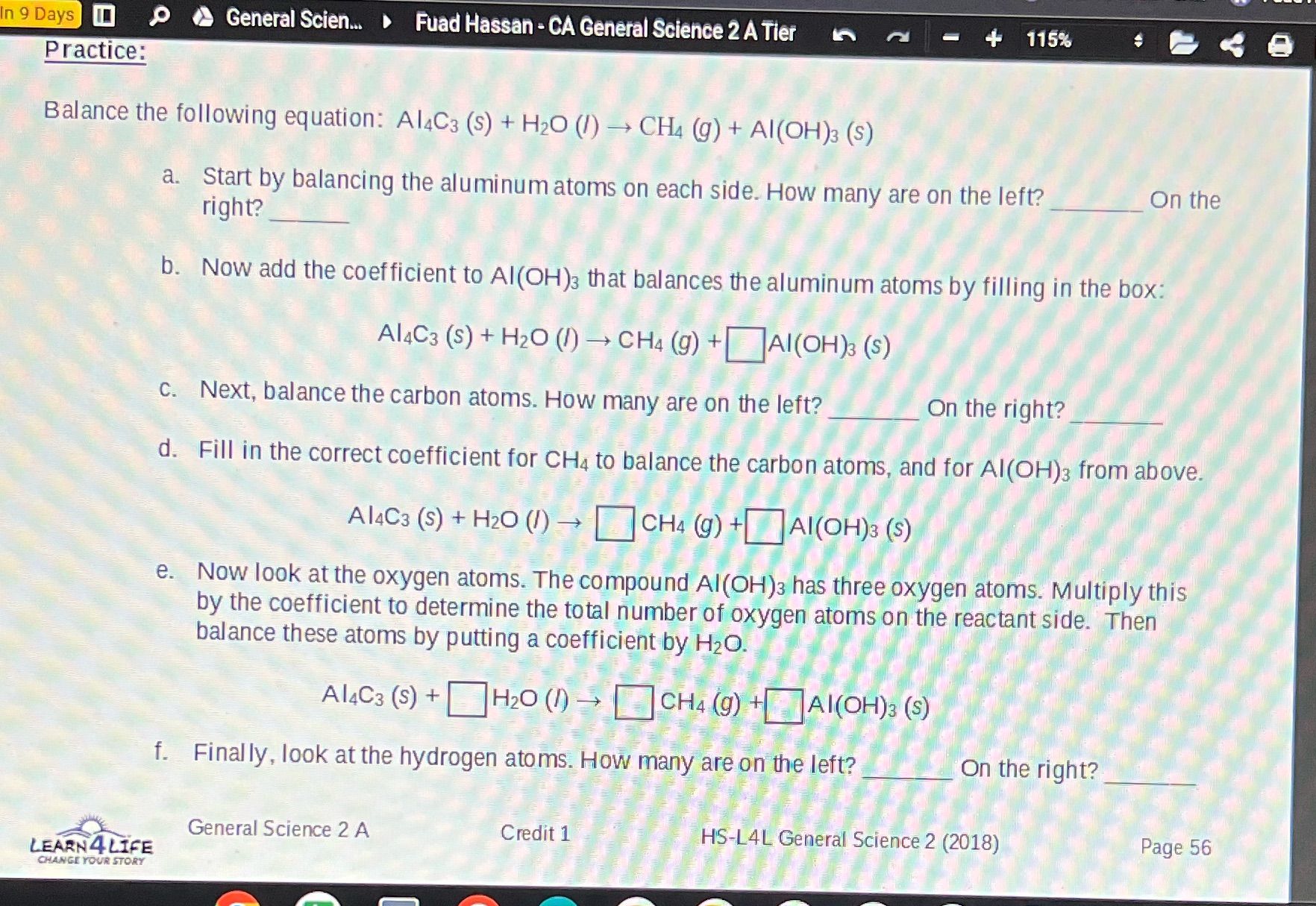
In 9 Days 0 General Scien... Fuad Hassan - CA General Science 2 A Tier Practice: 115% Balance the following equation: Al4C3 (s) + H2O (l) CH4 (g) + Al(OH)3 (S) a. Start by balancing the aluminum atoms on each side. How many are on the left? right? On the b. Now add the coefficient to Al(OH)3 that balances the aluminum atoms by filling in the box: Al4C3 (s) + H2O (1) CH4 (g) + Al(OH)3 (S) c. Next, balance the carbon atoms. How many are on the left? On the right? d. Fill in the correct coefficient for CH4 to balance the carbon atoms, and for Al(OH)3 from above. A14C3 (s) + H2O (1) CH4 (g) +Al(OH)3 (S) e. Now look at the oxygen atoms. The compound Al(OH)3 has three oxygen atoms. Multiply this by the coefficient to determine the total number of oxygen atoms on the reactant side. Then balance these atoms by putting a coefficient by HO. Al4C3 (s) + H2O (1) CH4 (g) +Al(OH)3 (s) f. Finally, look at the hydrogen atoms. How many are on the left? General Science 2 A LEARN 4 LIFE CHANGE YOUR STORY Credit 1 On the right? HS-L4L General Science 2 (2018) Page 56
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


