Question: In a CH4 molecule, each covalent bond forms by the overlap of which two atomic orbitals? C(sp2) and H(1s) C(sp3) and H(sp) C(sp3) and H(1s)
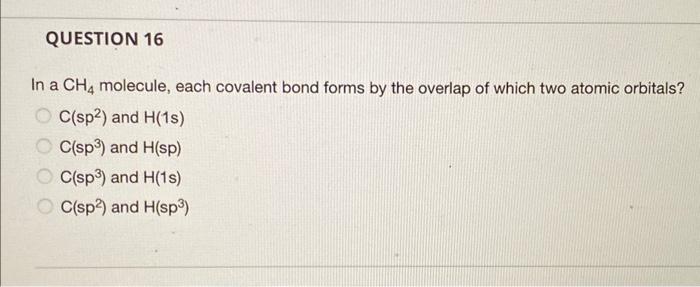
In a CH4 molecule, each covalent bond forms by the overlap of which two atomic orbitals? C(sp2) and H(1s) C(sp3) and H(sp) C(sp3) and H(1s) C(sp2) and H(sp3)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


