Question: In a closed container, under constant pressure, a liquid mixture of cyclohexane and toluene is evaporated. xc is the mole fraction of cyclohexane in the
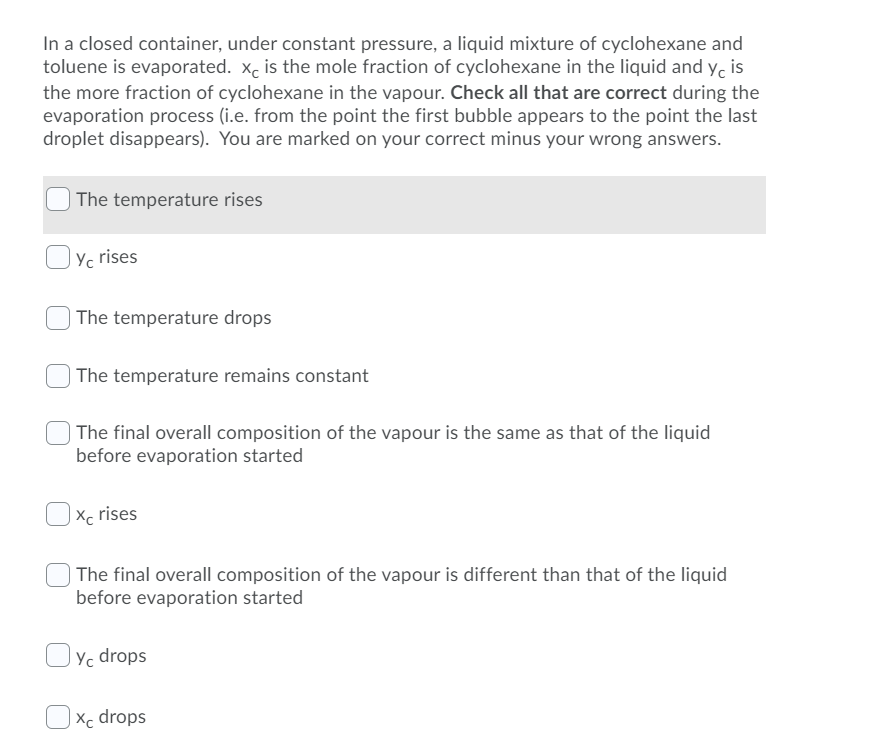
In a closed container, under constant pressure, a liquid mixture of cyclohexane and toluene is evaporated. xc is the mole fraction of cyclohexane in the liquid and yc is the more fraction of cyclohexane in the vapour. Check all that are correct during the evaporation process (i.e. from the point the first bubble appears to the point the last droplet disappears). You are marked on your correct minus your wrong answers. The temperature rises Yc rises The temperature drops The temperature remains constant The final overall composition of the vapour is the same as that of the liquid before evaporation started Xc rises The final overall composition of the vapour is different than that of the liquid before evaporation started Oy, drops Xc drops
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


