Question: In a laboratory experiment, a student found that a solution consisting of 22.38 g of an unknown compound (a nonvolatile nonelectrolyte) and 180.6 g of
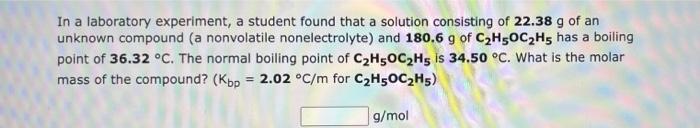
In a laboratory experiment, a student found that a solution consisting of 22.38 g of an unknown compound (a nonvolatile nonelectrolyte) and 180.6 g of C2H5OC2H5 has a boiling point of 36.32 C. The normal boiling point of C2H5OC2H5 is 34.50 C. What is the molar mass of the compound? (Kbp = 2.02 C/m for C2H5OC2H5) g/mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


