Question: In a non-flow piston Cylinder system working fluid # at 0.8 bar occupying 0.09m3 is compressed reversibly and Process 1 # to a pressure of
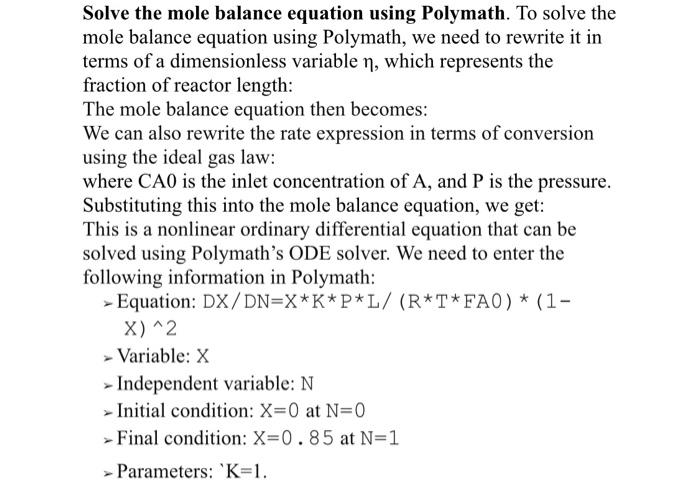
In a non-flow piston Cylinder system working fluid \# at 0.8 bar occupying 0.09m3 is compressed reversibly and Process 1 \# to a pressure of 5 bar. The working fluid \# is then heated reversibly and isochorically until the pressure is 7 bar; the specific volume is then 0.5m3/kg. A reversible isothermal expansion restores the working fluid \# to its initial state. i. Present the processes in a piston cylinder diagram for different stages ii. Plot the cycle on PV diagram and Label it properly iii. Examine the cycle and quantify the mass of the fluid and the temperature at each point of the cycle. iv. Analyse the different processes involved in the system by calculating the work done in individual process and the net work done in the cycle v. Analyse the heat supplied or rejected during each process using non flow energy equation Solve the mole balance equation using Polymath. To solve the mole balance equation using Polymath, we need to rewrite it in terms of a dimensionless variable , which represents the fraction of reactor length: The mole balance equation then becomes: We can also rewrite the rate expression in terms of conversion using the ideal gas law: where CA0 is the inlet concentration of A, and P is the pressure. Substituting this into the mole balance equation, we get: This is a nonlinear ordinary differential equation that can be solved using Polymath's ODE solver. We need to enter the following information in Polymath: - Equation: DX/DN=XKPL/(RTFAO)(1 x) 2 - Variable: X - Independent variable: N - Initial condition: X=0 at N=0 > Final condition: X=0.85 at N=1 > Parameters: ' K=1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


