Question: Examine the below scenario and find the Amounts of reactants used and products obtained Cyclohexanol Cyclohexene 100.158 Product theoretical yield (g) Product percent yield (%)
Examine the below scenario and find the Amounts of reactants used and products obtained
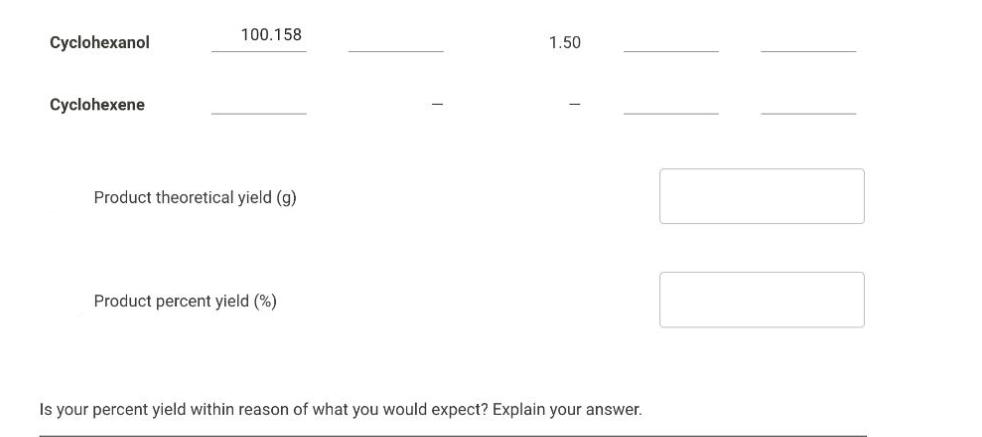
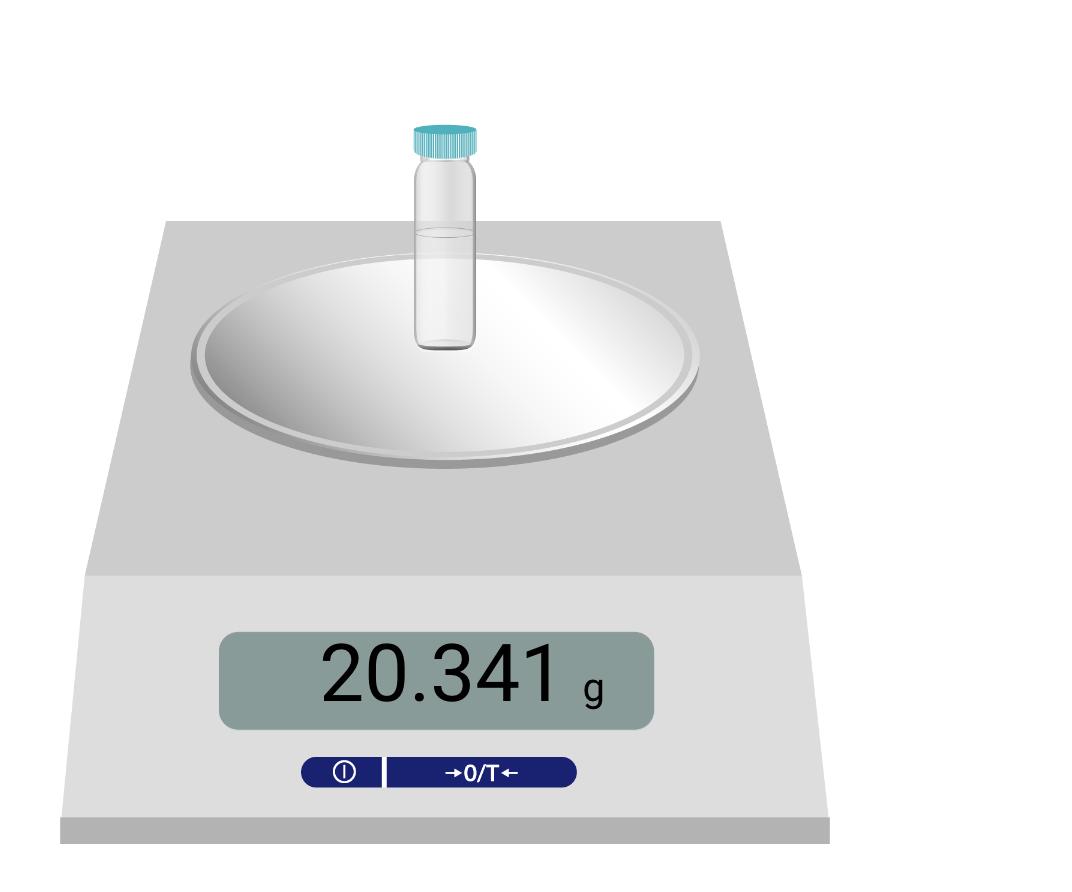
Cyclohexanol Cyclohexene 100.158 Product theoretical yield (g) Product percent yield (%) 1.50 Is your percent yield within reason of what you would expect? Explain your answer. LUM Cyclohexanol Cyclohexene 100.158 Product theoretical yield (g) Product percent yield (%) 1.50 Is your percent yield within reason of what you would expect? Explain your answer. LUM
Step by Step Solution
There are 3 Steps involved in it
ANSWER Density of cyclohexanol 962kgm3 By multiplying by 0001 we can convert th... View full answer

Get step-by-step solutions from verified subject matter experts


