Question: In a system as illustrated in a figure below, 10.0 kg mass falls for 3.00 m, resulting work. The work is used to turn a
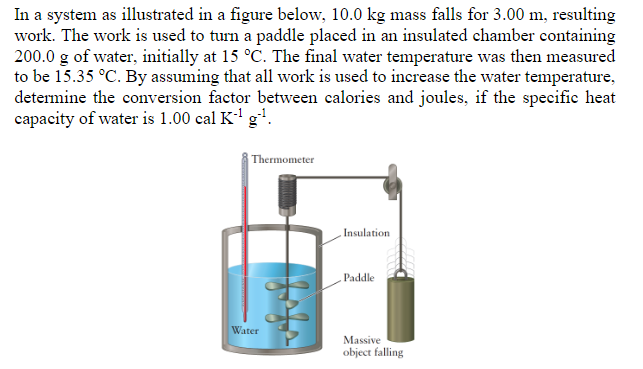
In a system as illustrated in a figure below, 10.0 kg mass falls for 3.00 m, resulting work. The work is used to turn a paddle placed in an insulated chamber containing 200.0 g of water, initially at 15 C. The final water temperature was then measured to be 15.35 C. By assuming that all work is used to increase the water temperature, determine the conversion factor between calories and joules, if the specific heat capacity of water is 1.00 cal K+g? Thermometer Insulation Paddle Water Massive object falling
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


