Question: In a titrimetric method, the following replicate end-points were obtained: 10.0,9.9, 9.8, 9.7 & 9.2 ml. A) Apply the Q-test to determine whether the outlying
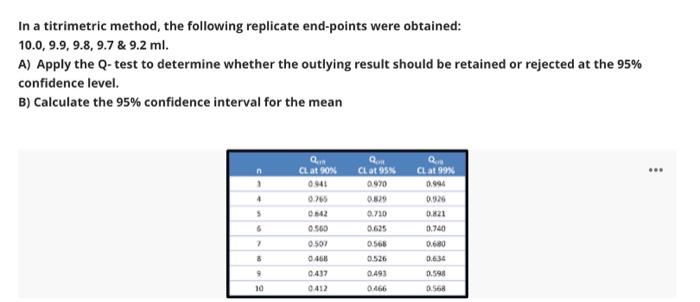




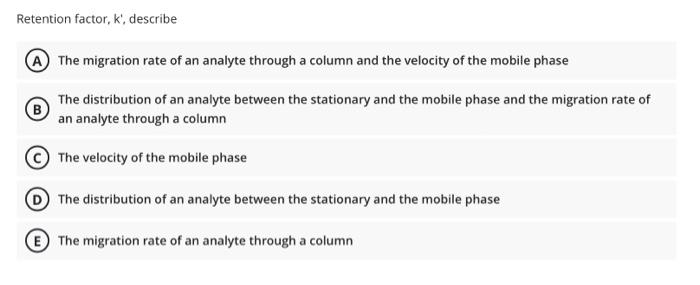
In a titrimetric method, the following replicate end-points were obtained: 10.0,9.9, 9.8, 9.7 & 9.2 ml. A) Apply the Q-test to determine whether the outlying result should be retained or rejected at the 95% confidence level. B) Calculate the 95% confidence interval for the mean QUIR Q n 1 + CL. at 90% 0441 0.265 Q CL at 95% 0.970 0.829 0.710 CL at 99 0.994 0.926 0221 0.740 0.680 5 6 0.560 0507 7 3 0.566 0.526 0.466 063 9 0.493 0598 0.437 0412 10 0466 0.568 Question 10 0.5 Points Accuracy and precision have the same meaning in analytical chemistry A True False The effect of a constant error becomes more serious as the size of the quantity measured decreases. A True (B) False Chromatography is the process for identification, purification and separation of components of a mixture on the basis of Difference in their solubility. A True B) False If the degree of supersaturation is (Q) and the solubility of precipitate is (S); then, According to Von Wiemarn equation, the relative supersaturation equals to: (Q-Sy/S B (S-1)/S (Q-5)/Q (S-2)/Q E (S-Qy/S Retention factor, k, describe A The migration rate of an analyte through a column and the velocity of the mobile phase B The distribution of an analyte between the stationary and the mobile phase and the migration rate of an analyte through a column The velocity of the mobile phase The distribution of an analyte between the stationary and the mobile phase E The migration rate of an analyte through a column
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


