Question: In a voltaic cell with a postive cell potential, the spontaneous reaction that occurs is Zn(s)+Ni2+Ni(s)+Zn2+ Consider the following three statements and choose the correct
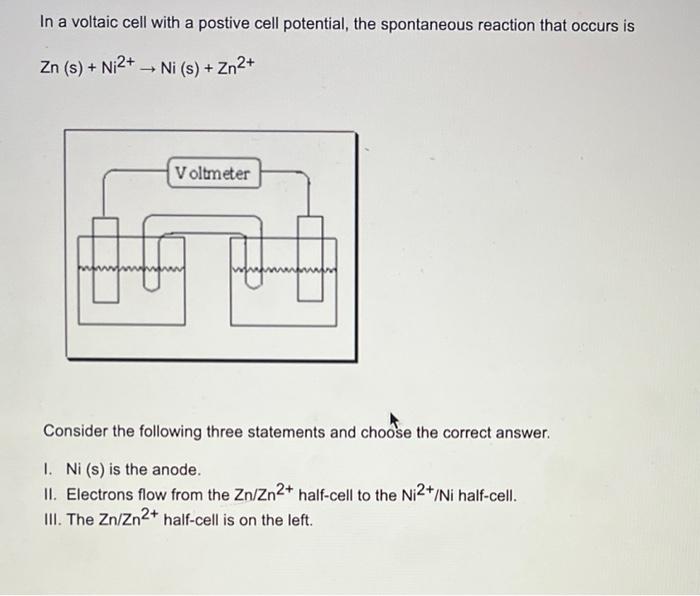
In a voltaic cell with a postive cell potential, the spontaneous reaction that occurs is Zn(s)+Ni2+Ni(s)+Zn2+ Consider the following three statements and choose the correct answer. I. Ni(s) is the anode. II. Electrons flow from the Zn/Zn2+ half-cell to the Ni2+/Ni half-cell. III. The Zn/Zn2+ half-cell is on the left
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


