Question: In an aqueous chloride solution cobalt ( II ) exists in equilibrium with the complex ion C o C l 4 2 - * C
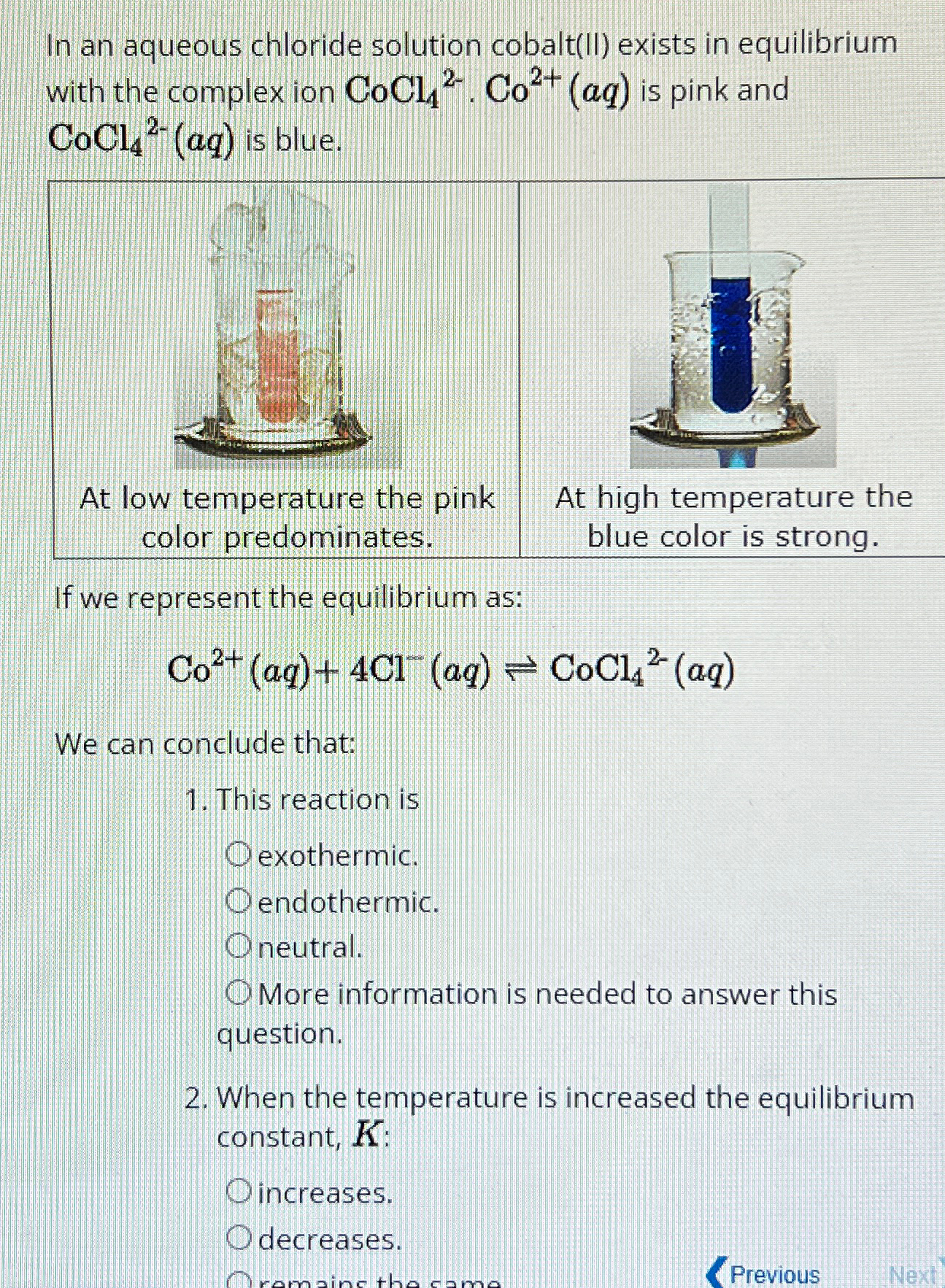
In an aqueous chloride solution cobaltII exists in equilibrium with the complex ion is pink and is blue.
At low temperature the pink At high temperature the color predominates. blue color is strong.
If we represent the equilibrium as:
We can conclude that:
This reaction is
exothermic.
endothermic.
neutral.
More information is needed to answer this question.
When the temperature is increased the equilibrium constant, :
increases.
decreases.
Previous
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


