Question: In an example problem on the water-gas shift reaction CO+H2O=CO2+H2, we found the temperature where the reaction is no longer spontaneous by assuming Hr and
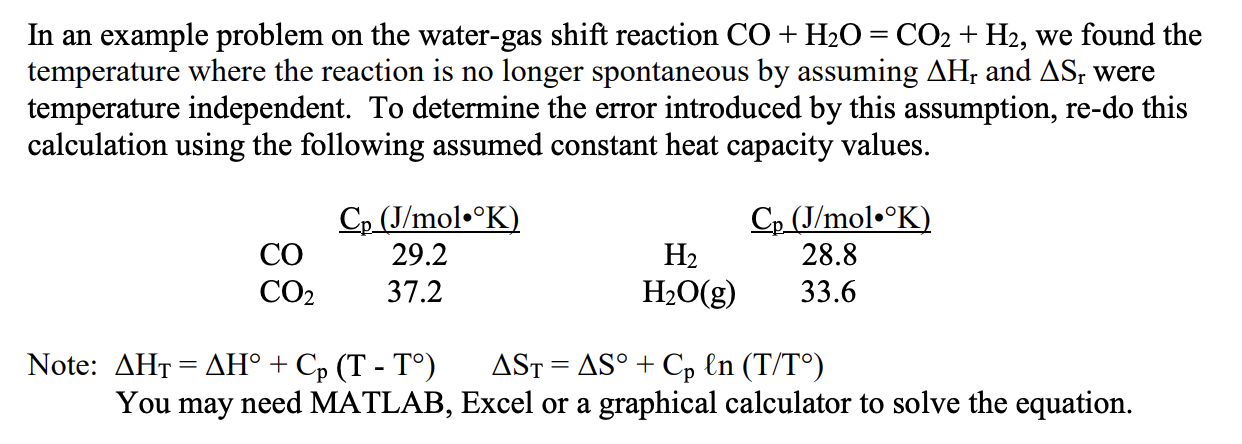
In an example problem on the water-gas shift reaction CO+H2O=CO2+H2, we found the temperature where the reaction is no longer spontaneous by assuming Hr and Sr were temperature independent. To determine the error introduced by this assumption, re-do this calculation using the following assumed constant heat capacity values. Note: HT=H+Cp(TT)ST=S+Cpln(T/T) You may need MATLAB, Excel or a graphical calculator to solve the equation
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


