Question: in c++ please answer questions and explain how you solved same equation used in the photoelectric effect. If these equations are combined, the wavelength of
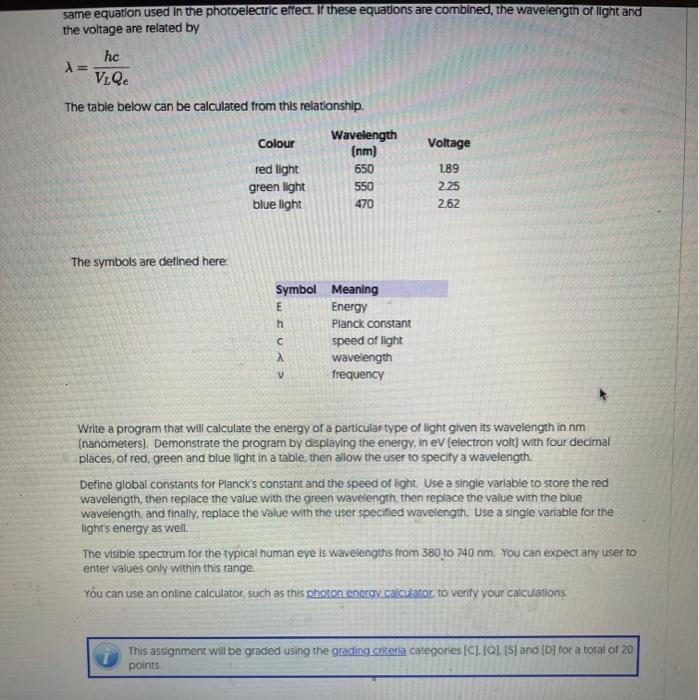
same equation used in the photoelectric effect. If these equations are combined, the wavelength of light and the voltage are related by hc X= V.Qe The table below can be calculated from this relationship. Colour red light green light blue light Wavelength (nm) 650 550 470 Voltage 1.89 2.25 2.62 The symbols are defined here CO Symbol Meaning E Energy h Planck constant speed of light wavelength frequency Write a program that will calculate the energy of a particular type of light given its wavelength in nm (nanometers). Demonstrate the program by displaying the energy, in eV (electron volt) with four decimal places, of red, green and blue light in a table, then allow the user to specity a wavelength Define global constants for Planck's constant and the speed of light. Use a single variable to store the red wavelength, then replace the value with the green wavelength, then replace the value with the blue wavelength, and finally, replace the value with the user specified wavelength. Use a single variable for the light's energy as well The visible spectrum for the typical human eye is wavelengths from 380 to 740 nm. You can expect any user to enter values only within this range You can use an online calculator, such as this photon energy calculator to verify your calculations This assignment will be graded using the grading criteria categories ICI.Q.IS) and ID for a total of 20 points
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


