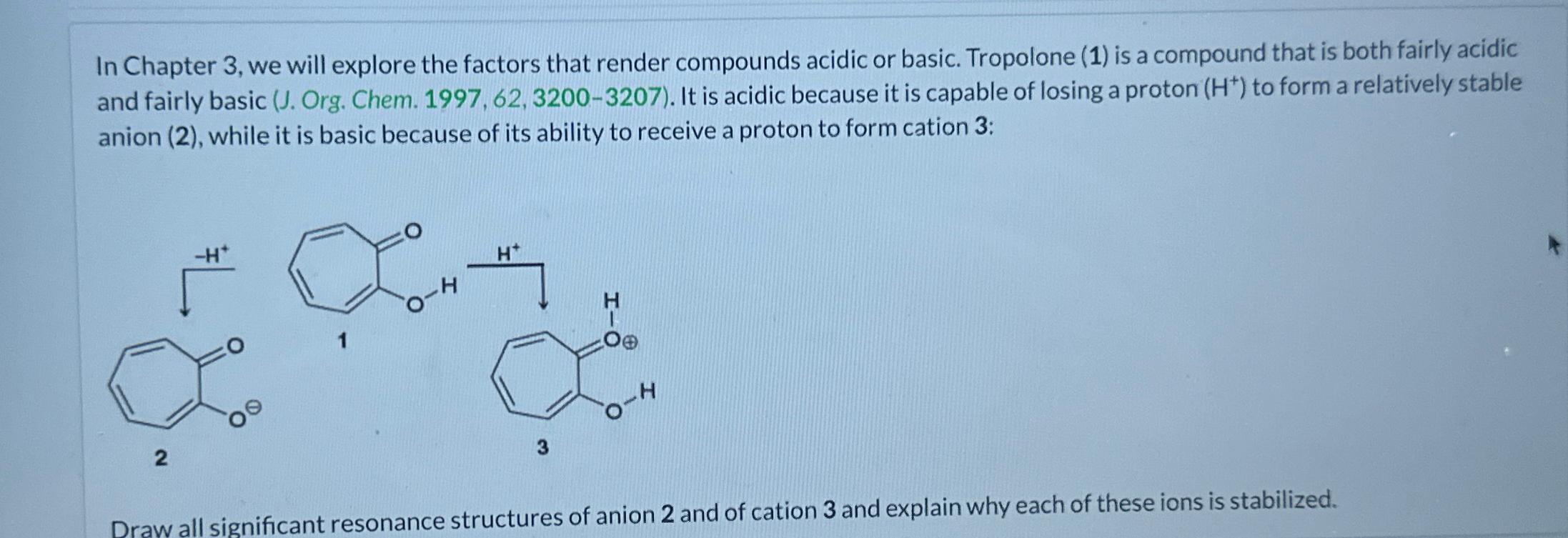
Question: In Chapter 3 , we will explore the factors that render compounds acidic or basic. Tropolone ( 1 ) is a compound that is both
In Chapter we will explore the factors that render compounds acidic or basic. Tropolone is a compound that is both fairly acidic and fairly basic J Org. Chem. It is acidic because it is capable of losing a proton to form a relatively stable anion while it is basic because of its ability to receive a proton to form cation :
Draw all significant resonance structures of anion and of cation and explain why each of these ions is stabilized.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


