Question: in handwriting please 2. Write balanced net ionic equations fort he following reactions. (20 marks) (a) SCN + Bro, Br + S0.2- + HCN (b)
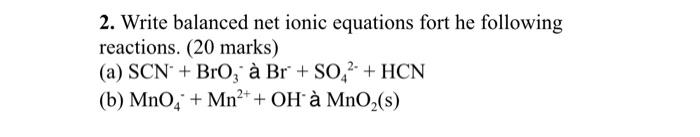
2. Write balanced net ionic equations fort he following reactions. (20 marks) (a) SCN + Bro, Br + S0.2- + HCN (b) MnO4 + Mn2+ + OH MnO2(s)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


