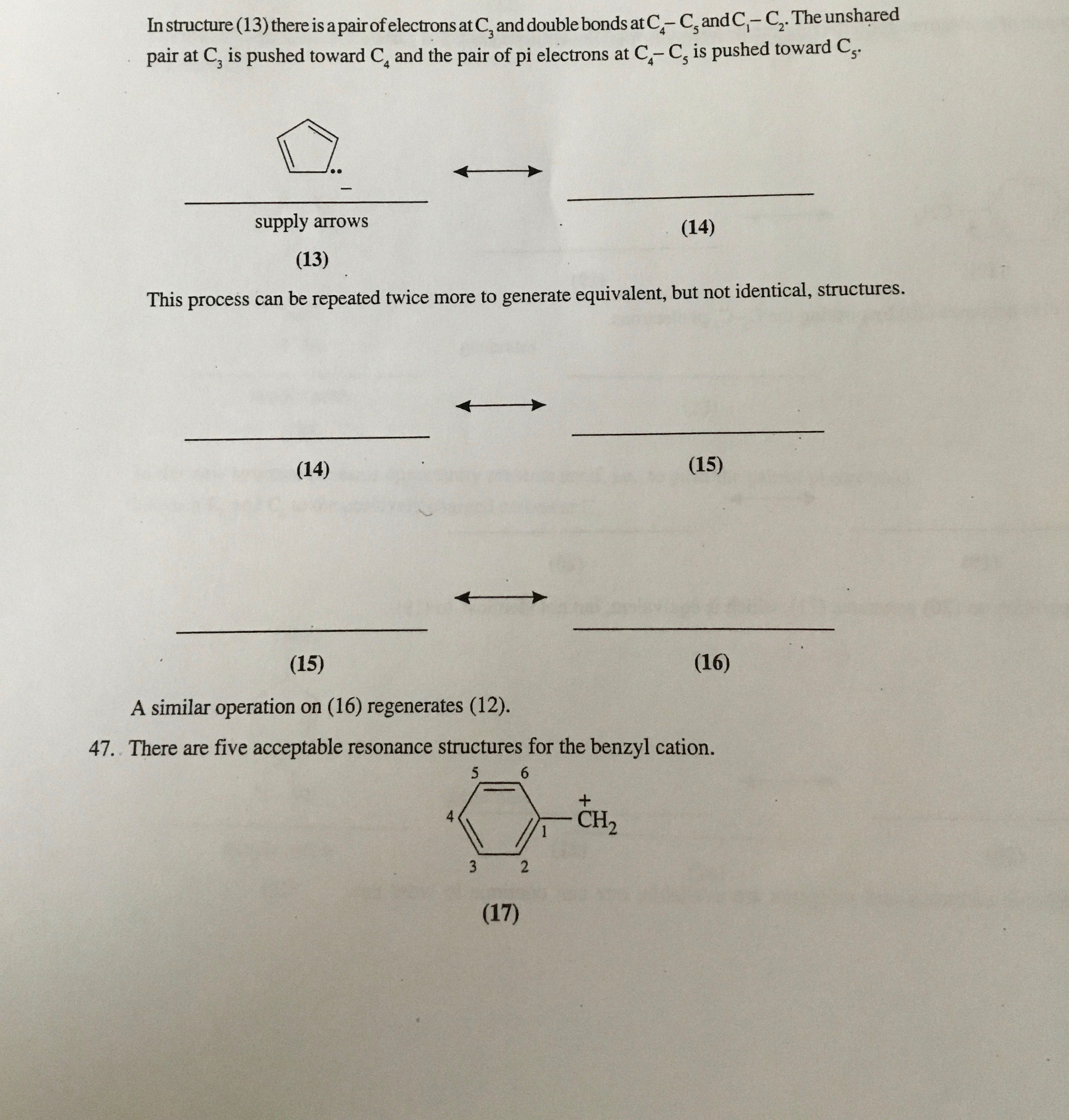
Question: In structure ( 1 3 ) there is a pair of electrons at C 3 and double bonds at C 4 - C 5 and
In structure there is a pair of electrons at and double bonds at and The unshared
pair at is pushed toward and the pair of pi electrons at is pushed toward
This process can be repeated twice more to generate equivalent, but not identical, structures.
A similar operation on regenerates
There are five acceptable resonance structures for the benzyl cation.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


