Question: in the book given point B with out mention temperature how much how drawn line and lever arm explain please by pencil not typing A
in the book given point B with out mention temperature how much how drawn line and lever arm explain please by pencil not typing 
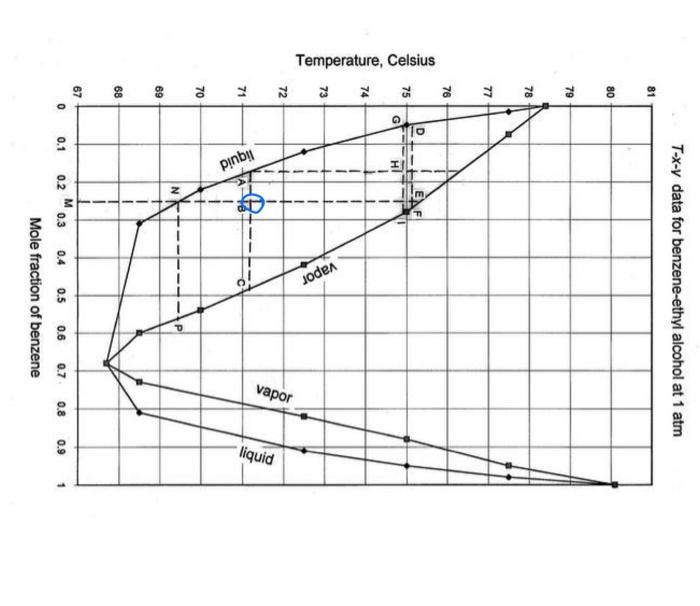


A liquid mixture containing 25mol% benzene and 75mol% ethyl alcohol, in which components are miscible in all proportions, is heated at a constant pressure of 1atm from 60C to 90C. Using the following Txy experimental data, determine (a) the temperature where vaporization begins; (b) the composition of the first bubble of vapor; (c) the composition of the residual liquid when 25mol \% has evaporated, assuming that all vapor formed is retained in the apparatus and is in equilibrium with the residual liquid. (d) Repeat Temperature, Celsius
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


