Question: In the paper manufacturing process, different processes are used to produce wood pulps from trees. One process of interest is the sulfite process. You will

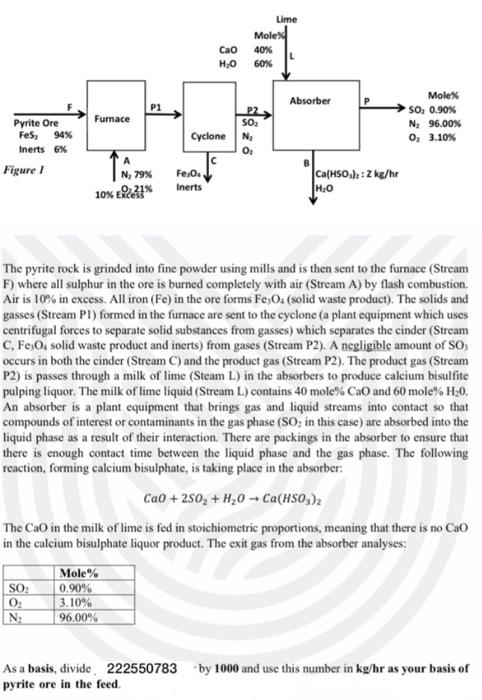
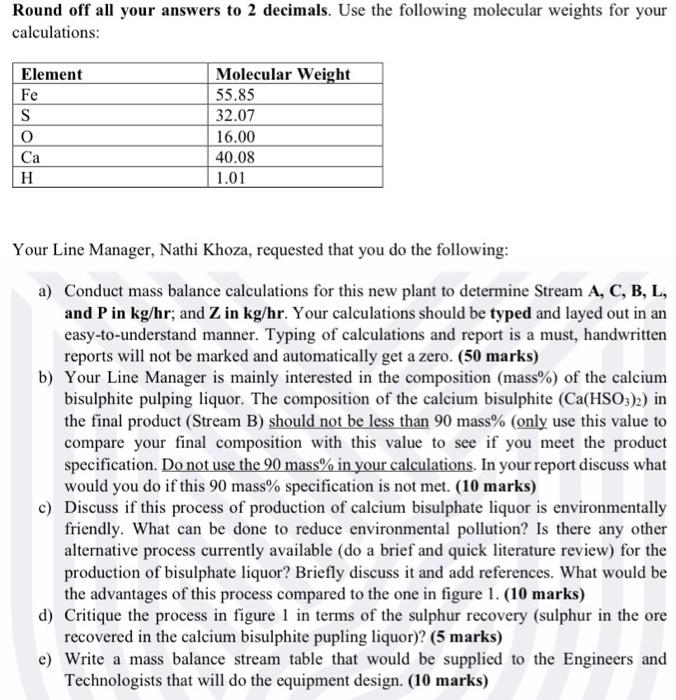
In the paper manufacturing process, different processes are used to produce wood pulps from trees. One process of interest is the sulfite process. You will learn about the other processes in Chemical Process Industries (EHCPI1A). The sulfite process produces wood pulp that is almost pure cellulose fibers by treating wood chips with solutions of sulphite and bisulphite ions. You have been employed for your Work Integrated Learning (WIL) at SAPPI in Ngodwana in the Mpumalanga Province. Due to an increase in the price of bisulphite pulping liquor that they were purchasing from another company, they are in the process of building a new plant that will produce this sulphite pulping liquor. There is a local mine, Ngodwana Iron Ore Mine, that is mining the mineral pyrite ore. The desired reactant in the pyrites ore is FeS2. The new plant will use pyrite ore as a source of SO2 required for the production of bisulphite pulping liquor. SO2 is produce in the furnace, where FeS2 is burned with air as per the following balanced reaction: 3FeS2+8O2Fe3O4+6SO2 The pyrite ore from Ngodwana Iron Ore Mine contains on average 94%FeS2 and 6% inerts on a weight basis. The proposed block flow diagram of the new bisulphite pulping liquor is shown in Figure 1. The pyrite rock is grinded into fine powder using mills and is then sent to the furnace (Stream F) where all sulphur in the ore is burned completely with air (Stream A) by flash combustion. Air is 10% in excess. All iron (Fe) in the ore forms Fe3O4 (solid waste product). The solids and gasses (Stream P1) formed in the furnace are sent to the cyclone (a plant equipment which uses centrifugal forces to separate solid substances from gasses) which separates the cinder (Stream C,Fe3O4 solid waste product and inerts) from gases (Stream P2 ). A negligible amount of SO3 occurs in both the cinder (Stream C) and the product gas (Stream P2). The product gas (Stream P2) is passes through a milk of lime (Steam L) in the absorbers to produce calcium bisulfite pulping liquor. The milk of lime liquid (Stream L) contains 40 mole\% CaO and 60 mole %H20. An absorber is a plant equipment that brings gas and liquid streams into contact so that compounds of interest or contaminants in the gas phase (SO2 in this case) are absorbed into the liquid phase as a result of their interaction. There are packings in the absorber to ensure that there is enough contact time between the liquid phase and the gas phase. The following reaction, forming calcium bisulphate, is taking place in the absorber: CaO+2SO2+H2OCa(HSO3)2 The CaO in the milk of lime is fed in stoichiometric proportions, meaning that there is no CaO in the calcium bisulphate liquor product. The exit gas from the absorber analyses: As a basis, divide. 222550783 by 1000 and use this number in kg/hr as your basis of pyrite ore in the feed. Round off all your answers to 2 decimals. Use the following molecular weights for your calculations: Your Line Manager, Nathi Khoza, requested that you do the following: a) Conduct mass balance calculations for this new plant to determine StreamA,C,B,L, and P in kg/hr; and Z in kg/hr. Your calculations should be typed and layed out in an easy-to-understand manner. Typing of calculations and report is a must, handwritten reports will not be marked and automatically get a zero. (50 marks) b) Your Line Manager is mainly interested in the composition (mass\%) of the calcium bisulphite pulping liquor. The composition of the calcium bisulphite (Ca(HSO3)2) in the final product (Stream B) should not be less than 90 mass\% (only use this value to compare your final composition with this value to see if you meet the product specification. Do not use the 90 mass % in your calculations. In your report discuss what would you do if this 90 mass % specification is not met. (10 marks) c) Discuss if this process of production of calcium bisulphate liquor is environmentally friendly. What can be done to reduce environmental pollution? Is there any other alternative process currently available (do a brief and quick literature review) for the production of bisulphate liquor? Briefly discuss it and add references. What would be the advantages of this process compared to the one in figure 1. (10 marks) d) Critique the process in figure 1 in terms of the sulphur recovery (sulphur in the ore recovered in the calcium bisulphite pupling liquor)? (5 marks) e) Write a mass balance stream table that would be supplied to the Engineers and Technologists that will do the equipment design. (10 marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


