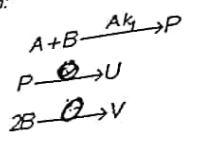
Question: in the reaction scheme: selectivity is defined as: consider that E1 >E2>E3 (Ei = activation energy of reaction i, (1,2,3)) and k2=0. What conditions lead
in the reaction scheme:
selectivity is defined as:
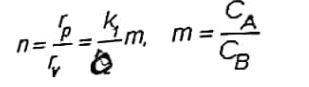
consider that E1 >E2>E3 (Ei = activation energy of reaction i, (1,2,3)) and k2=0. What conditions lead to greater selectivity to obtain the maximum benefit?
a) High P and High T
b) High mass and high n
c) High T and high n
d) High T, Excess of CA
Please explain why
Aky P F A+B. 28 11 E || =0
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


