Question: In the recent article L i 2 G e S 3 : Lithium Ionic Conductor with an Unprecedented Structural Type ( Inorganic Chemistry 2 0
In the recent article : Lithium Ionic Conductor with an Unprecedented Structural
Type Inorganic Chemistry ; doi acsinorgchemc
Hong et al describe a new material for LithiumIon Batteries LIBs Despite a
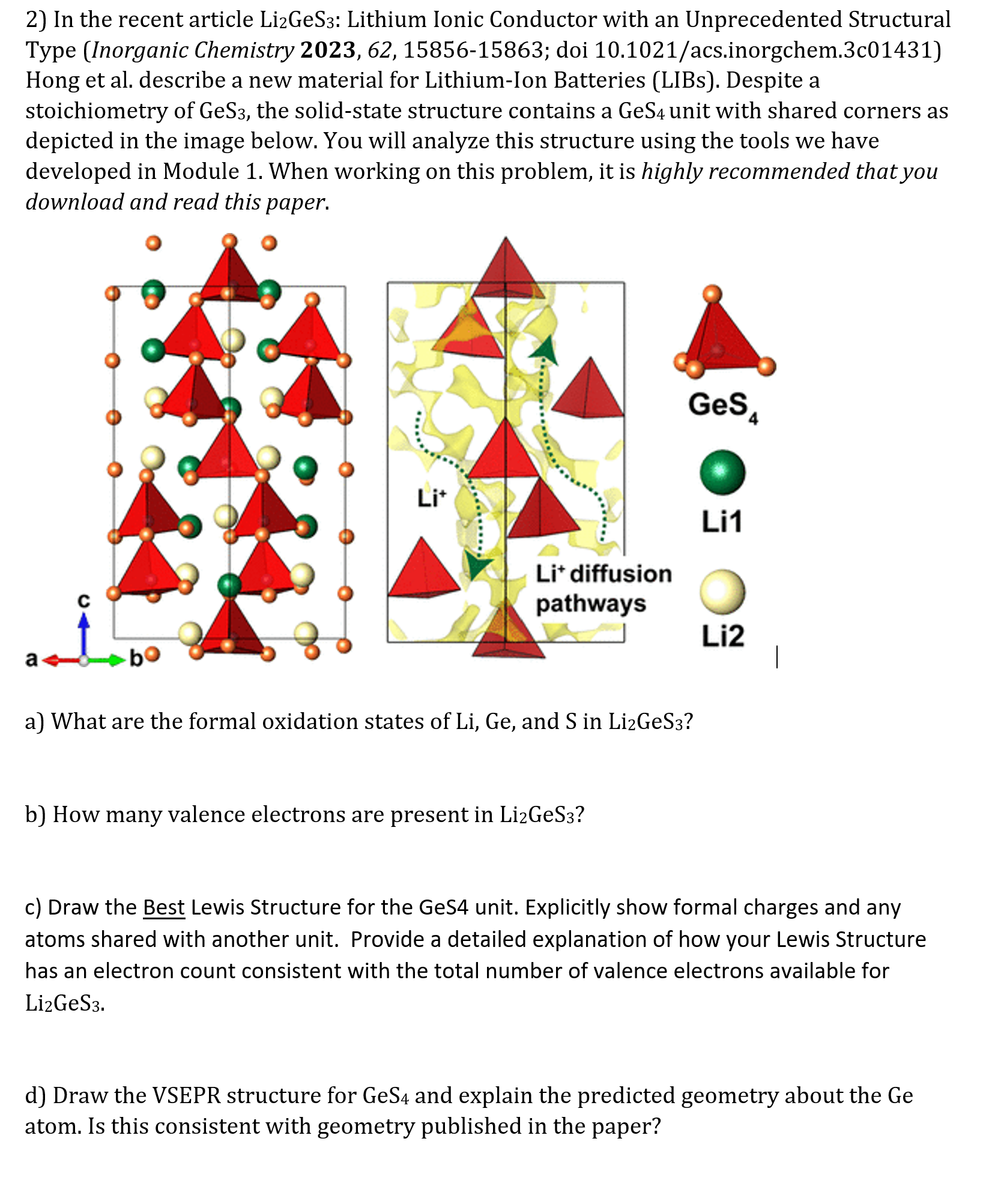
stoichiometry of the solidstate structure contains a unit with shared corners as
depicted in the image below. You will analyze this structure using the tools we have
developed in Module When working on this problem, it is highly recommended that you
download and read this paper.
Li
a What are the formal oxidation states of and in
b How many valence electrons are present in
c Draw the Best Lewis Structure for the GeS unit. Explicitly show formal charges and any
atoms shared with another unit. Provide a detailed explanation of how your Lewis Structure
has an electron count consistent with the total number of valence electrons available for
d Draw the VSEPR structure for and explain the predicted geometry about the Ge
atom. Is this consistent with geometry published in the paper?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


