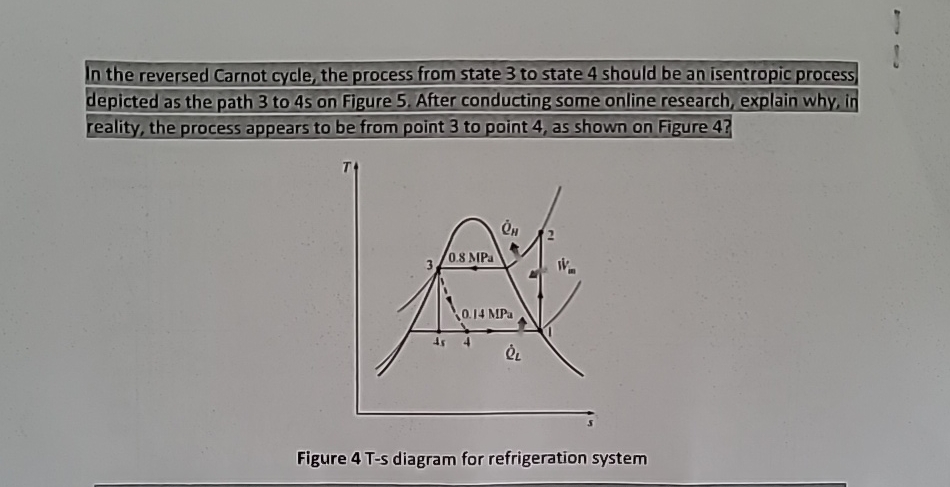
Question: In the reversed Carnot cycle, the process from state 3 to state 4 should be an isentropic process, depicted as the path 3 to 4
In the reversed Carnot cycle, the process from state to state should be an isentropic process, depicted as the path to s on Figure After conducting some online research, explain why, in reality, the process appears to be from point to point as shown on Figure
Figure Ts diagram for refrigeration system
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


