Question: In this problem, we will explore the process of free expansion. Consider a rigid container that is closed, insulated from its surroundings, and divided into
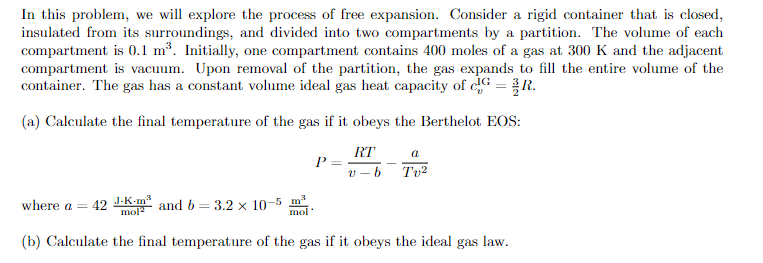
In this problem, we will explore the process of free expansion. Consider a rigid container that is closed, insulated from its surroundings, and divided into two compartments by a partition. The volume of each compartment is 0.1 m?. Initially, one compartment contains 400 moles of a gas at 300 K and the adjacent compartment is vacuum. Upon removal of the partition, the gas expands to fill the entire volume of the container. The gas has a constant volume ideal gas heat capacity of G = R. (a) Calculate the final temperature of the gas if it obeys the Berthelot EOS: P RT U-b a Tv2 where a = 42 J.Kom" and b=3.2 x 10-5 m mol (b) Calculate the final temperature of the gas if it obeys the ideal gas law
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


