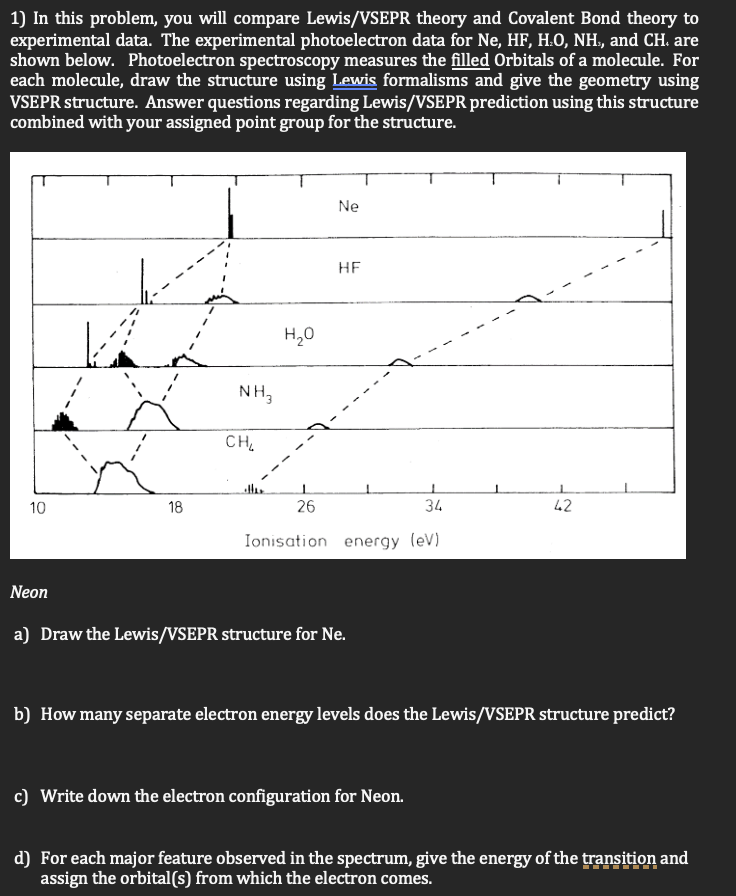
Question: In this problem, you will compare Lewis / VSEPR theory and Covalent Bond theory to experimental data. The experimental photoelectron data for N e ,
In this problem, you will compare LewisVSEPR theory and Covalent Bond theory to experimental data. The experimental photoelectron data for and are shown below. Photoelectron spectroscopy measures the filled Orbitals of a molecule. For each molecule, draw the structure using Lewis formalisms and give the geometry using VSEPR structure. Answer questions regarding LewisVSEPR prediction using this structure combined with your assigned point group for the structure.
Neon
a Draw the LewisVSEPR structure for
b How many separate electron energy levels does the LewisVSEPR structure predict?
c Write down the electron configuration for Neon.
d For each major feature observed in the spectrum, give the energy of the transition and assign the orbitals from which the electron comes.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


