Question: incorrect. Briefly discuss why H2 S has a lower boiling point than H2Se. Select all that apply. H2S has less dispersion forces than H2Se H2S
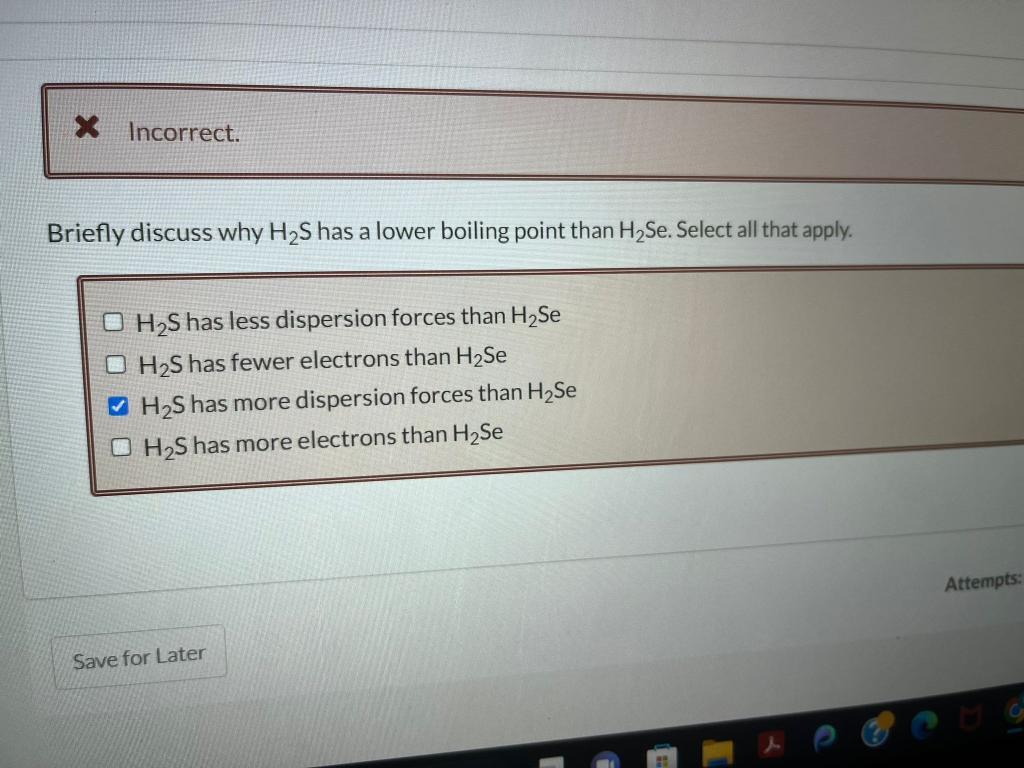
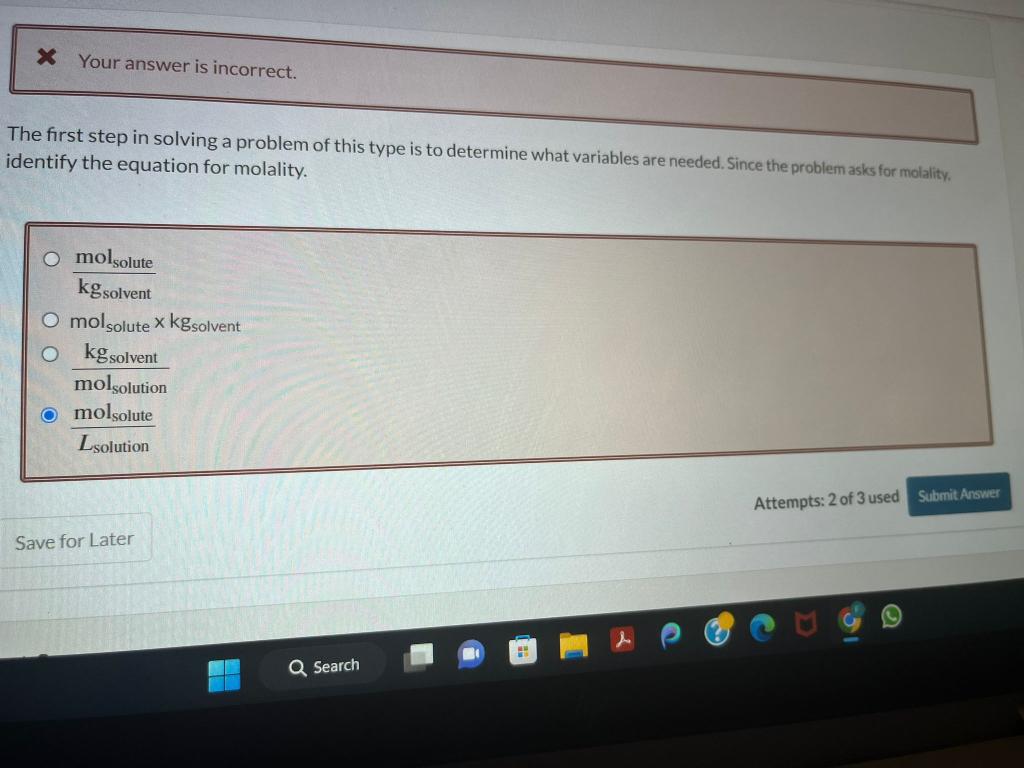
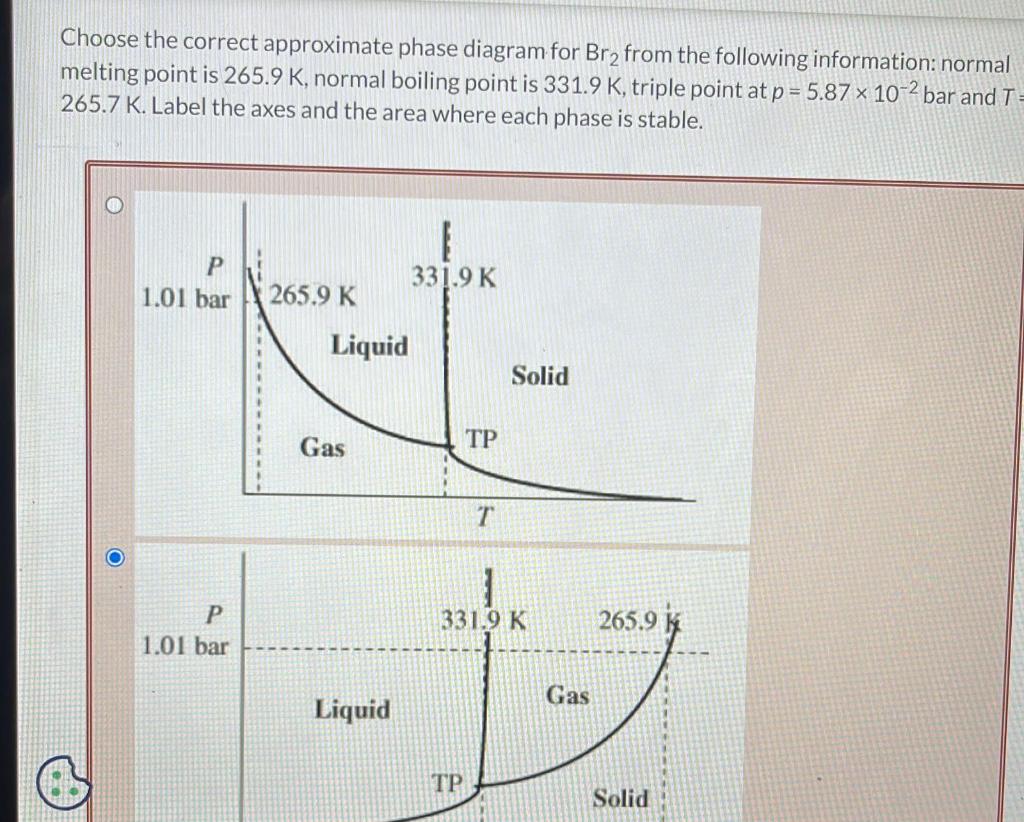
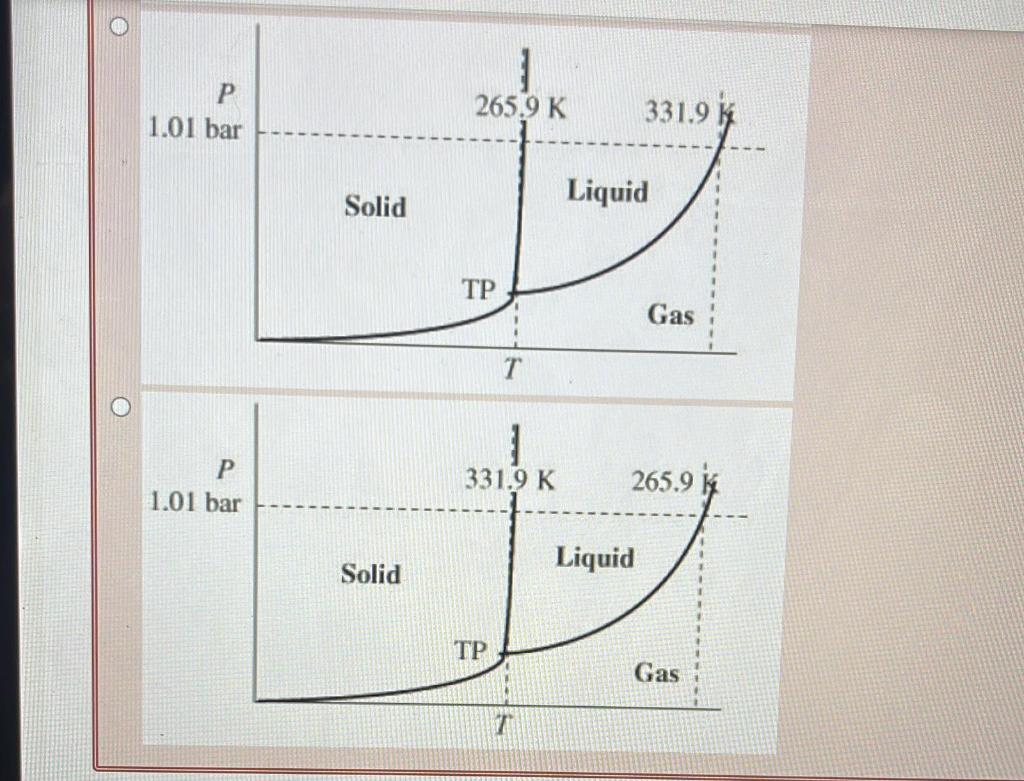
incorrect. Briefly discuss why H2 S has a lower boiling point than H2Se. Select all that apply. H2S has less dispersion forces than H2Se H2S has fewer electrons than H2Se H2S has more dispersion forces than H2Se H2S has more electrons than H2Se is Your answer is incorrect. The first step in solving a problem of this type is to determine what variables are needed. Since the problem asks for molality. dentify the equation for molality. kgsolventmolsolute molsolutexkgsolvent molsolutionkgsolvent Lsolutionmolsolute Choose the correct approximate phase diagram for Br2 from the following information: normal melting point is 265.9K, normal boiling point is 331.9K, triple point at p=5.87102 bar and T= 265.7 K. Label the axes and the area where each phase is stable
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


