Question: Indicate whether the molecules shown below are aromatic, non-aromatic or anti-aromatic. Some molecules have an aromatic/anti-aromatic region and a non-aromatic region. If this is the
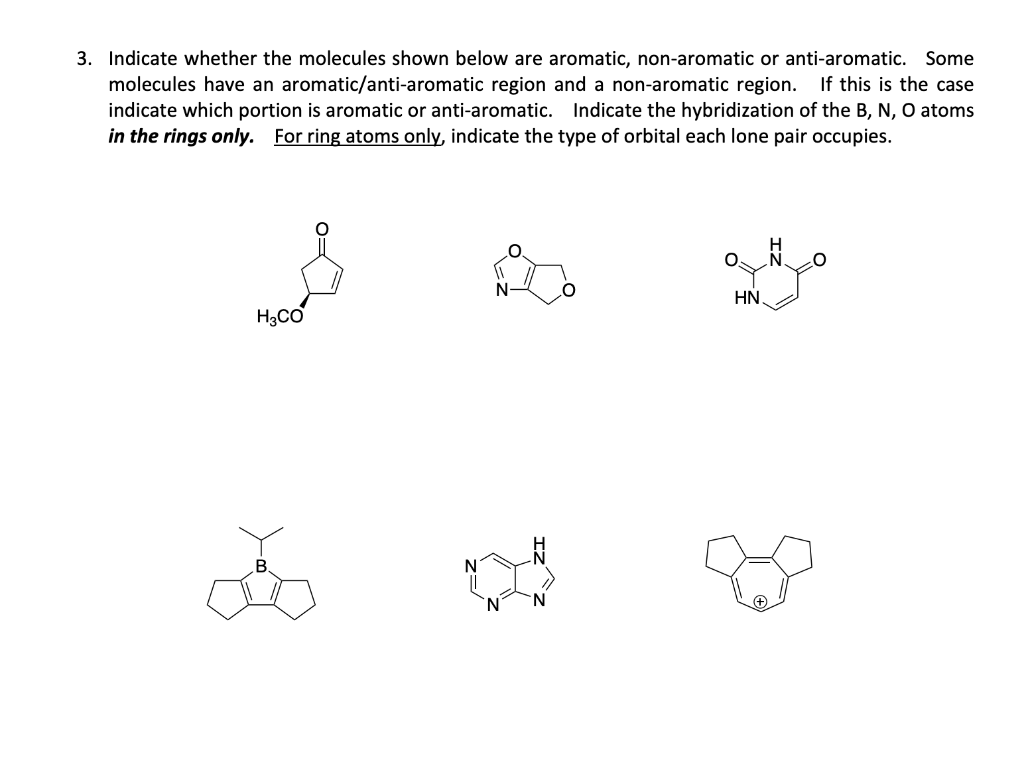
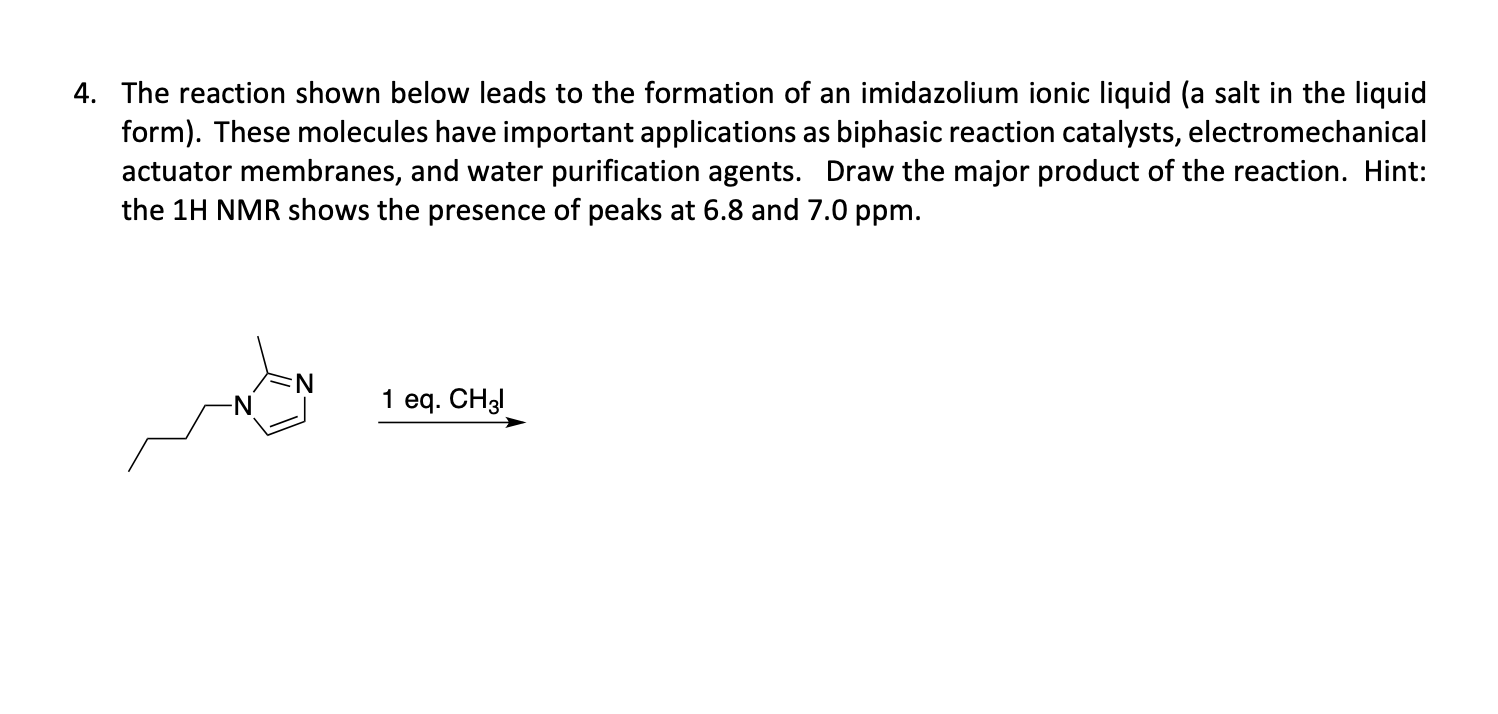
Indicate whether the molecules shown below are aromatic, non-aromatic or anti-aromatic. Some molecules have an aromatic/anti-aromatic region and a non-aromatic region. If this is the case indicate which portion is aromatic or anti-aromatic. Indicate the hybridization of the B, N, O atoms in the rings only. For ring atoms only, indicate the type of orbital each lone pair occupies. 4. The reaction shown below leads to the formation of an imidazolium ionic liquid (a salt in the liquid form). These molecules have important applications as biphasic reaction catalysts, electromechanical actuator membranes, and water purification agents. Draw the major product of the reaction. Hint: the 1H NMR shows the presence of peaks at 6.8 and 7.0ppm. 1eq.CH3I
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


