Question: Indicate with a + or a - which atom is more electropositive and which is more electronegative. Then, using Linus Pauling's numbers, subtract their electronegativities
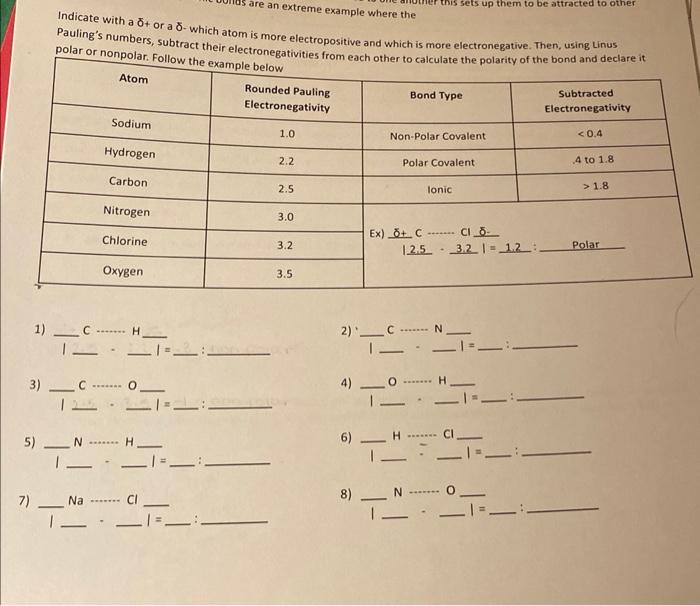
Indicate with a + or a - which atom is more electropositive and which is more electronegative. Then, using Linus Pauling's numbers, subtract their electronegativities from each other to calculate the nolaritv of the bond and declare it polar or nonpolar. Follow tha 1) 1C+H1H 2). CCIN 3) 1C110 4) IOH 5) N ...... H H= 6) 7) NaCl 8) 11N
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


