Question: Initially, at a temperature T1 and a molar volume v1, a van der Waals gas undergoes a change of state to the final temperature
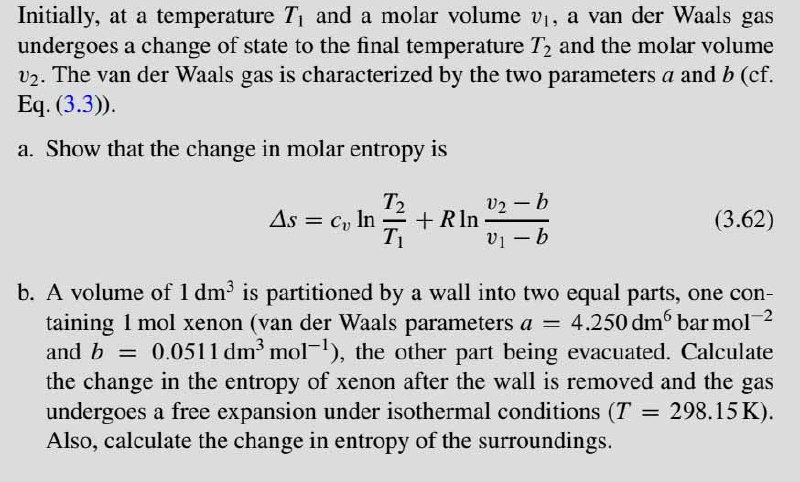
Initially, at a temperature T1 and a molar volume v1, a van der Waals gas undergoes a change of state to the final temperature T2 and the molar volume v2. The van der Waals gas is characterized by the two parameters a and b (cf. Eq. (3.3)). a. Show that the change in molar entropy is V2 - b + Rln v1 b T2 As = C, In (3.62) b. A volume of 1 dm is partitioned by a wall into two equal parts, one con- 4.250 dm bar mol 2 taining 1 mol xenon (van der Waals parameters a = and b = 0.0511 dm' mol-), the other part being evacuated. Calculate the change in the entropy of xenon after the wall is removed and the gas undergoes a free expansion under isothermal conditions (T = 298.15 K). Also, calculate the change in entropy of the surroundings. %3D
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


