Question: Calculate the composition (mole fractions) of the product stream arising from the adiabatic combustion of Octane (CSH18) burning stoichiometrically in air, with the assumption
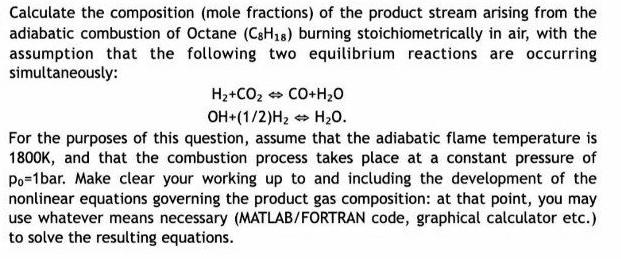
Calculate the composition (mole fractions) of the product stream arising from the adiabatic combustion of Octane (CSH18) burning stoichiometrically in air, with the assumption that the following two equilibrium reactions are occurring simultaneously: H2+CO2 CO+H20 OH+(1/2)H2 H20. For the purposes of this question, assume that the adiabatic flame temperature is 1800K, and that the combustion process takes place at a constant pressure of Po=1bar. Make clear your working up to and including the development of the nonlinear equations governing the product gas composition: at that point, you may use whatever means necessary (MATLAB/FORTRAN code, graphical calculator etc.) to solve the resulting equations.
Step by Step Solution
3.49 Rating (149 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


