Question: Initial-rate data at a certain temperature is given in the table for the reaction CHCl(g) CH(g)+HCl(g) [CHCI], (M) Initial rate (M/s) 0.100 0.770 x 10-30
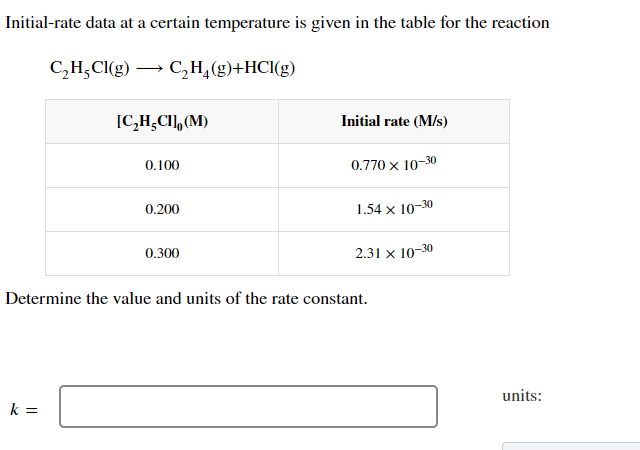
Initial-rate data at a certain temperature is given in the table for the reaction CHCl(g) CH(g)+HCl(g) [CHCI], (M) Initial rate (M/s) 0.100 0.770 x 10-30 0.200 1.54 x 10-30 0.300 2.31 x 10-30 Determine the value and units of the rate constant. k= units
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


