Question: inneed help with # 2. d please 2) Using symmetry arguments, indicate whether each of the following compounds should/should not possess a molecular dipole moment:
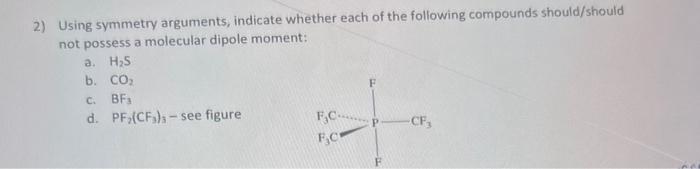
2) Using symmetry arguments, indicate whether each of the following compounds should/should not possess a molecular dipole moment: a. H2S b. CO2 c. BF3 d. PF2(CF3)3 see figure
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


